Title 49
SECTION 173.199
173.199 Category B infectious substances.
§ 173.199 Category B infectious substances.(a) Category B infectious substances. Except as provided in this paragraph (a), Category B infectious substances are excepted from all other requirements of this subchapter when offered for transportation or transported in accordance with this section. Category B infectious substances offered for transportation or transported under the provisions of this section are subject to the incident reporting requirements in §§ 171.15 and 171.16 of this subchapter and to the requirements in § 175.75(b) of this subchapter concerning cargo location. Except as provided in paragraph (a)(9) of this section, a Category B infectious substance meeting the definition of a hazard class other than Division 6.2 must be offered for transportation or transported in accordance with applicable requirements of this subchapter.
(1) A Category B infectious substance must be packaged in a triple packaging consisting of a primary receptacle, a secondary packaging, and a rigid outer packaging.
(2) Primary receptacles must be packed in secondary packaging in such a way that, under normal conditions of transport, they cannot break, be punctured, or leak their contents into the secondary packaging.
(3) Secondary packagings must be secured in rigid outer packagings with suitable cushioning material such that any leakage of the contents will not impair the protective properties of the cushioning material or the outer packaging.
(4) The completed package must be designed, constructed, maintained, filled, its contents limited, and closed so that under conditions normally encountered in transportation, including removal from a pallet or overpack for subsequent handling, there will be no release of hazardous material into the environment. Package effectiveness must not be substantially reduced for minimum and maximum temperatures, changes in humidity and pressure, and shocks, loadings and vibrations normally encountered during transportation. The packaging must be capable of successfully passing the drop test in § 178.609(d) of this subchapter at a drop height of at least 1.2 meters (3.9 feet). Following the drop test, there must be no leakage from the primary receptacle, which must remain protected by absorbent material, when required, in the secondary packaging. At least one surface of the outer packaging must have a minimum dimension of 100 mm by 100 mm (3.9 inches).
(5) The following square-on-point mark must be displayed on the outer packaging on a background of contrasting color. The width of the line forming the border must be at least 2 mm (0.08 inches) and the letters and numbers must be at least 6 mm (0.24 inches) high. The size of the mark must be such that no side of the diamond is less than 50 mm (1.97 inches) in length as measured from the outside of the lines forming the border. The proper shipping name “Biological substances, Category B” must be marked on the outer packaging adjacent to the diamond-shaped mark in letters that are at least 6 mm (0.24 inches) high.
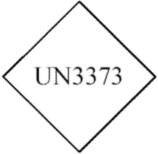
(i) Transitional exception - A marking in conformance with the requirements of this paragraph in effect on December 31, 2014, may continue to be used until December 31, 2016.
(ii) For domestic transportation, a packaging marked prior to January 1, 2017 and in conformance with the requirements of this paragraph in effect on December 31, 2014, may continue in service until the end of its useful life.
(6) When packages are placed in an overpack, the package markings required by this section must be either clearly visible or reproduced on the outside of the overpack.
(7) The name and telephone number of a person who is either knowledgeable about the material being shipped and has comprehensive emergency response and incident mitigation information for the material, or has immediate access to a person who possesses such knowledge and information, must be included on a written document (such as an air waybill or bill of lading) or on the outer packaging. The telephone number must be monitored during a company's administrative hours (i.e., company's operational business hours).
(8) For transportation by aircraft, each package, overpack, pallet, or unit load device containing a Category B infectious substance must be inspected for leakage when it is unloaded from the aircraft. If evidence of leakage is found, the cargo compartment in which the package, overpack, pallet, or unit load device was transported must be disinfected. Disinfection may be by any means that will make the material released ineffective at transmitting disease.
(9) A packaging containing inner packagings of Category B infectious substances may not contain other hazardous materials except -
(i) Refrigerants, such as dry ice or liquid nitrogen, as authorized under paragraph (d) of this section;
(ii) Anticoagulants used to stabilize blood or plasma; or
(iii) Small quantities of Class 3, Class 8, Class 9, or other materials in Packing Groups II and III used to stabilize or prevent degradation of the sample, provided the quantity of such materials does not exceed 30 mL (1 ounce) or 30 g (1 ounce) in each inner packaging. Such preservatives are not subject to the requirements of this subchapter.
(10) Clear instructions on filling and closing a packaging used to transport a Category B infectious substance must be provided by the packaging manufacturer and subsequent distributors to the consignor or person who prepares the package to enable the package to be correctly prepared for transport. A copy or electronic image of these instructions must be retained by the manufacturer and subsequent distributors for at least one year from the date of issuance, and made available for inspection by a Federal or state government representative upon request. Packagings must be filled and closed in accordance with the information provided by the packaging manufacturer or subsequent distributor.
(b) Liquid Category B infectious substances. Liquid Category B infectious substances must be packaged in conformance with the following provisions:
(1) The primary receptacle must be leakproof.
(2) Absorbent material must be placed between the primary receptacle and secondary packaging. If several fragile primary receptacles are placed in a single secondary packaging, they must be either individually wrapped or separated to prevent contact between them. The absorbent material must be of sufficient quantity to absorb the entire contents of the primary receptacles and not compromise the integrity of the cushioning material or the outer packaging.
(3) The secondary packaging must be leakproof.
(4) For shipments by aircraft, the primary receptacle or the secondary packaging must be capable of withstanding without leakage an internal pressure producing a pressure differential of not less than 95 kPa (0.95 bar, 14 psi).
(5) For shipments by aircraft, the maximum quantity contained in each primary receptacle, including any material used to stabilize or prevent degradation of the sample, may not exceed 1 L (34 ounces), and the maximum quantity contained in each outer packaging, including any material used to stabilize or prevent degradation of the samples, may not exceed 4 L (1 gallon). The outer packaging limitation does not include ice, dry ice, or liquid nitrogen when used to maintain the integrity of the material.
(c) Solid Category B infectious substances. Solid Category B infectious substances must be packaged in a triple packaging, consisting of a primary receptacle, secondary packaging, and outer packaging, conforming to the following provisions:
(1) The primary receptacle must be siftproof.
(2) If several fragile primary receptacles are placed in a single secondary packaging, they must be either individually wrapped or separated to prevent contact between them.
(3) The secondary packaging must be siftproof.
(4) If residual liquid may be present in the primary receptacle during transportation, then the material must be transported in accordance with requirements in paragraph (b) of this section. A solid material that may become liquid during transportation must be transported in accordance with paragraph (b) of this section.
(5) Except for packages containing body parts, organs, or whole bodies, for shipment by aircraft, the outer packaging may not contain more than 4 kg (8.8 pounds), including any material used to stabilize or prevent degradation of the samples. The outer packaging limitation does not include ice, dry ice, or liquid nitrogen when used to maintain the integrity of the material.
(d) Refrigerated or frozen specimens (ice, dry ice, and liquid nitrogen). In addition to complying with the requirements in this paragraph (d), dry ice and liquid nitrogen must be offered for transportation or transported in accordance with the applicable requirements of this subchapter.
(1) Ice or dry ice must be placed outside the secondary packaging or in an overpack. Interior supports must be provided to secure the secondary packagings in the original position. If ice is used, the outside packaging must be leakproof or must have a leakproof liner. If dry ice is used, the outside packaging must permit the release of carbon dioxide gas and otherwise meet the provisions in § 173.217. The primary receptacle and secondary packaging must maintain their integrity at the temperature of the refrigerant used, as well as the temperatures and pressures of transport by aircraft they could be subjected to if refrigeration were lost, and sufficient absorbent material must be provided to absorb all liquid, including melted ice.
(2) The package is marked “Carbon dioxide, solid” or “Dry ice” and an indication that the material being refrigerated is used for diagnostic or treatment purposes (e.g., frozen medical specimens).
(e) Training. Each person who offers or transports a Category B infectious substance under the provisions of this section must know about the requirements of this section.
[67 FR 53142, Aug. 14, 2002, as amended at 71 FR 32261, June 2, 2006; 72 FR 55693, Oct. 1, 2007; 78 FR 1088, Jan. 7, 2013; 80 FR 1160, Jan. 8, 2015; 80 FR 72927, Nov. 23, 2015; 81 FR 35542, June 2, 2016; 85 FR 83399, Dec. 21, 2020]