Title 40
SECTION 53.61
53.61 Test conditions.
§ 53.61 Test conditions.(a) Sampler surface preparation. Internal surfaces of the candidate sampler shall be cleaned and dried prior to performing any Class II sampler test in this subpart. The internal collection surfaces of the sampler shall then be prepared in strict accordance with the operating instructions specified in the sampler's operating manual referred to in section 7.4.18 of 40 CFR part 50, appendix L.
(b) Sampler setup. Set up and start up of all test samplers shall be in strict accordance with the operating instructions specified in the manual referred to in section 7.4.18 of 40 CFR part 50, appendix L, unless otherwise specified within this subpart.
(c) Sampler adjustments. Once the test sampler or samplers have been set up and the performance tests started, manual adjustment shall be permitted only between test points for all applicable tests. Manual adjustments and any periodic maintenance shall be limited to only those procedures prescribed in the manual referred to in section 7.4.18 of 40 CFR part 50, appendix L. The submitted records shall clearly indicate when any manual adjustment or periodic maintenance was made and shall describe the operations performed.
(d) Sampler malfunctions. If a test sampler malfunctions during any of the applicable tests, that test run shall be repeated. A detailed explanation of all malfunctions and the remedial actions taken shall be submitted as part of the equivalent method application.
(e) Particle concentration measurements. All measurements of particle concentration must be made such that the relative error in measurement is less than 5.0 percent. Relative error is defined as (s × 100 percent)/(X), where s is the sample standard deviation of the particle concentration detector, X is the measured concentration, and the units of s and X are identical.
(f) Operation of test measurement equipment. All test measurement equipment shall be set up, calibrated, and maintained by qualified personnel according to the manufacturer's instructions. All appropriate calibration information and manuals for this equipment shall be kept on file.
(g) Vibrating orifice aerosol generator conventions. This section prescribes conventions regarding the use of the vibrating orifice aerosol generator (VOAG) for the size-selective performance tests outlined in §§ 53.62, 53.63, 53.64, and 53.65.
(1) Particle aerodynamic diameter. The VOAG produces near-monodisperse droplets through the controlled breakup of a liquid jet. When the liquid solution consists of a non-volatile solute dissolved in a volatile solvent, the droplets dry to form particles of near-monodisperse size.
(i) The physical diameter of a generated spherical particle can be calculated from the operating parameters of the VOAG as:
Equation 1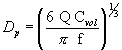 where: Dp =
particle physical diameter, µm; Q = liquid volumetric flow rate, µm
3/sec; Cvol = volume concentration (particle volume produced per
drop volume), dimensionless; and f = frequency of applied
vibrational signal, 1/sec.
where: Dp =
particle physical diameter, µm; Q = liquid volumetric flow rate, µm
3/sec; Cvol = volume concentration (particle volume produced per
drop volume), dimensionless; and f = frequency of applied
vibrational signal, 1/sec.
(ii) A given particle's aerodynamic behavior is a function of its physical particle size, particle shape, and density. Aerodynamic diameter is defined as the diameter of a unit density (ρo = 1g/cm 3) sphere having the same settling velocity as the particle under consideration. For converting a spherical particle of known density to aerodynamic diameter, the governing relationship is:
Equation 2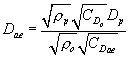 where: Dae =
particle aerodynamic diameter, µm; ρp = particle density, g/cm 3;
ρo = aerodynamic particle density = 1 g/cm 3; CDp = Cunningham's
slip correction factor for physical particle diameter,
dimensionless; and CDae = Cunningham's slip correction factor for
aerodynamic particle diameter, dimensionless.
where: Dae =
particle aerodynamic diameter, µm; ρp = particle density, g/cm 3;
ρo = aerodynamic particle density = 1 g/cm 3; CDp = Cunningham's
slip correction factor for physical particle diameter,
dimensionless; and CDae = Cunningham's slip correction factor for
aerodynamic particle diameter, dimensionless.
(iii) At room temperature and standard pressure, the Cunningham's slip correction factor is solely a function of particle diameter:
Equation 3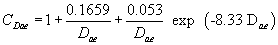 or Equation 4
or Equation 4
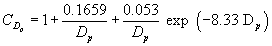
(iv) Since the slip correction factor is itself a function of particle diameter, the aerodynamic diameter in equation 2 of paragraph (g)(1)(ii) of this section cannot be solved directly but must be determined by iteration.
(2) Solid particle generation. (i) Solid particle tests performed in this subpart shall be conducted using particles composed of ammonium fluorescein. For use in the VOAG, liquid solutions of known volumetric concentration can be prepared by diluting fluorescein powder (C20H12O5, FW = 332.31, CAS 2321-07-5) with aqueous ammonia. Guidelines for preparation of fluorescein solutions of the desired volume concentration (Cvol) are presented by Vanderpool and Rubow (1988) (Reference 2 in appendix A of this subpart). For purposes of converting particle physical diameter to aerodynamic diameter, an ammonium fluorescein density of 1.35 g/cm 3 shall be used.
(ii) Mass deposits of ammonium fluorescein shall be extracted and analyzed using solutions of 0.01 N ammonium hydroxide.
(3) Liquid particle generation. (i) Tests prescribed in § 53.63 for inlet aspiration require the use of liquid particle tests composed of oleic acid tagged with uranine to enable subsequent fluorometric quantitation of collected aerosol mass deposits. Oleic acid (C18H34O2, FW = 282.47, CAS 112-80-1) has a density of 0.8935 g/cm 3. Because the viscosity of oleic acid is relatively high, significant errors can occur when dispensing oleic acid using volumetric pipettes. For this reason, it is recommended that oleic acid solutions be prepared by quantifying dispensed oleic acid gravimetrically. The volume of oleic acid dispensed can then be calculated simply by dividing the dispensed mass by the oleic acid density.
(ii) Oleic acid solutions tagged with uranine shall be prepared as follows. A known mass of oleic acid shall first be diluted using absolute ethanol. The desired mass of the uranine tag should then be diluted in a separate container using absolute ethanol. Uranine (C20H10O5Na2, FW = 376.3, CAS 518-47-8) is the disodium salt of fluorescein and has a density of 1.53 g/cm 3. In preparing uranine tagged oleic acid particles, the uranine content shall not exceed 20 percent on a mass basis. Once both oleic acid and uranine solutions are properly prepared, they can then be combined and diluted to final volume using absolute ethanol.
(iii) Calculation of the physical diameter of the particles produced by the VOAG requires knowledge of the liquid solution's volume concentration (Cvol). Because uranine is essentially insoluble in oleic acid, the total particle volume is the sum of the oleic acid volume and the uranine volume. The volume concentration of the liquid solution shall be calculated as:
Equation 5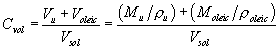 where: Vu =
uranine volume, ml; Voleic = oleic acid volume, ml; Vsol = total
solution volume, ml; Mu = uranine mass, g; ρu = uranine density,
g/cm 3; Moleic = oleic acid mass, g; and ρoleic = oleic acid
density, g/cm. 3
where: Vu =
uranine volume, ml; Voleic = oleic acid volume, ml; Vsol = total
solution volume, ml; Mu = uranine mass, g; ρu = uranine density,
g/cm 3; Moleic = oleic acid mass, g; and ρoleic = oleic acid
density, g/cm. 3
(iv) For purposes of converting the particles' physical diameter to aerodynamic diameter, the density of the generated particles shall be calculated as:
Equation 6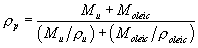
(v) Mass deposits of oleic acid shall be extracted and analyzed using solutions of 0.01 N sodium hydroxide.
[62 FR 38814, July 18, 1997; 63 FR 7714, Feb. 17, 1998]