Appendix A to Part 101 - Monier-Williams Procedure (With Modifications) for Sulfites in Food, Center for Food Safety and Applied Nutrition, Food and Drug Administration (November 1985)
21:2.0.1.1.2.9.1.1.1 : Appendix A
Appendix A to Part 101 - Monier-Williams Procedure (With
Modifications) for Sulfites in Food, Center for Food Safety and
Applied Nutrition, Food and Drug Administration (November 1985)
The AOAC official method for sulfites (Official Methods of
Analysis, 14th Edition, 20.123-20.125, AOAC INTERNATIONAL) has
been modified, in FDA laboratories, to facilitate the determination
of sulfites at or near 10 ppm in food. Method instructions,
including modifications, are described below.
Apparatus - The apparatus shown diagrammatically (Figure
1) is designed to accomplish the selective transfer of sulfur
dioxide from the sample in boiling aqueous hydrochloric acid to a
solution of 3% hydrogen peroxide. This apparatus is easier to
assemble than the official apparatus and the back pressure inside
the apparatus is limited to the unavoidable pressure due to the
height of the 3% H2O2 solution above the tip of the bubbler (F).
Keeping the backpressure as low as possible reduces the likelihood
that sulfur dioxide will be lost through leaks.
The apparatus should be assembled as shown in Fig. 1 with a thin
film of stopcock grease on the sealing surfaces of all the joints
except the joint between the separatory funnel and the flask. Each
joint should be clamped together to ensure a complete seal
throughout the analysis. The separatory funnel, B, should have a
capacity of 100 ml or greater. An inlet adapter, A, with a hose
connector (Kontes K-183000 or equivalent) is required to provide a
means of applying a head of pressure above the solution. (A
pressure equalizing dropping funnel is not recommended because
condensate, perhaps with sulfur dioxide, is deposited in the funnel
and the side arm.) The round bottom flask, C, is a 1000 ml flask
with three 24/40 tapered joints. The gas inlet tube, D, (Kontes
K-179000 or equivalent) should be of sufficient length to permit
introduction of the nitrogen within 2.5 cm of the bottom of the
flask. The Allihn condenser, E, (Kontes K-431000-2430 or
equivalent) has a jacket length of 300 mm. The bubbler, F, was
fabricated from glass according to the dimensions given in Fig. 2.
The 3% hydrogen peroxide solution can be contained in a vessel, G,
with an i.d. of ca. 2.5 cm and a depth of 18 cm.
Buret - A 10 ml buret (Fisher Cat. No. 03-848-2A or
equivalent) with overflow tube and hose connections for an Ascarite
tube or equivalent air scrubbing apparatus. This will permit the
maintenance of a carbon dioxide-free atmosphere over the
standardized 0.01N sodium hydroxide.
Chilled Water Circulator - The condensor must be chilled
with a coolant, such as 20% methanol-water, maintained at 5 °C. A
circulating pump equivalent to the Neslab Coolflow 33 is
suitable.
Reagents
(a) Aqueous hydrochloric acid, 4N. - For each analysis
prepare 90 ml of hydrochloric acid by adding 30 ml of concentrated
hydrochloric acid (12N) to 60 ml of distilled water.
(b) Methyl red indicator - Dissolve 250 mg of methyl red
in 100 ml ethanol.
(c) Hydrogen peroxide solution, 3% - Dilute ACS reagent
grade 30% hydrogen peroxide to 3% with distilled water. Just prior
to use, add three drops of methyl red indicator and titrate to a
yellow end-point using 0.01N sodium hydroxide. If the
end-point is exceeded discard the solution and prepare another 3%
H2O2 solution.
(d) Standardized titrant, 0.01N NaOH - Certified reagent
may be used (Fisher SO-5-284). It should be standardized with
reference standard potassium hydrogen phthalate.
(e) Nitrogen - A source of high purity nitrogen is
required with a flow regulator that will maintain a flow of 200 cc
per minute. To guard against the presence of oxygen in the
nitrogen, an oxygen scrubbing solution such as an alkaline
pyrogallol trap may be used. Prepare pyrogallol trap as
follows:
1. Add 4.5 g pyrogallol to the trap.
2. Purge trap with nitrogen for 2 to 3 minutes.
3. Prepare a KOH solution prepared by adding 65g KOH to 85 ml
distilled water (caution: heat).
4. Add the KOH solution to the trap while maintaining an
atmosphere of nitrogen in the trap.
Determination
Assemble the apparatus as shown in Fig. 1. The flask C must be
positioned in a heating mantle that is controlled by a power
regulating device such as Variac or equivalent. Add 400 ml of
distilled water to flask C. Close the stopcock of separatory
funnel, B, and add 90 ml of 4N hydrochloric acid to the
separatory funnel. Begin the flow of nitrogen at a rate of 200±10
cc/min. The condenser coolant flow must be initiated at this time.
Add 30 ml of 3% hydrogen peroxide, which has been titrated to a
yellow end-point with 0.01N NaOH, to container G. After
fifteen minutes the apparatus and the distilled water will be
thoroughly de-oxygenated and the apparatus is ready for sample
introduction.
Sample preparation (solids) - Transfer 50 g of food, or a
quantity of food with a convenient quantity of SO2 (500 to 1500 mcg
SO2), to a food processor or blender. Add 100 ml of 5% ethanol in
water and briefly grind the mixture. Grinding or blending should be
continued only until the food is chopped into pieces small enough
to pass through the 24/40 point of flask C.
Sample preparation (liquids) - Mix 50 g of the sample, or
a quantity with a convenient quantity of SO2 (500 to 1500 mcg SO2),
with 100 ml of 5% ethanol in water.
Sample introduction and distillation - Remove the
separatory funnel B, and quantitatively transfer the food sample in
aqueous ethanol to flask C. Wipe the tapered joint clean with a
laboratory tissue, apply stopcock grease to the outer joint of the
separatory funnel, and return the separatory funnel, B, to tapered
joint flask C. The nitrogen flow through the 3% hydrogen peroxide
solution should resume as soon as the funnel, B, is re-inserted
into the appropriate joint in flask C. Examine each joint to ensure
that it is sealed.
Apply a head pressure above the hydrochloric acid solution in B
with a rubber bulb equipped with a valve. Open the stopcock in B
and permit the hydrochloric acid solution to flow into flask C.
Continue to maintain sufficient pressure above the acid solution to
force the solution into the flask C. The stopcock may be closed, if
necessary, to pump up the pressure above the acid and then opened
again. Close the stopcock before the last few milliliters drain out
of the separatory funnel, B, to guard against the escape of sulfur
dioxide into the separatory funnel.
Apply the power to the heating mantle. Use a power setting which
will cause 80 to 90 drops per minute of condensate to return to the
flask from condenser, E. After 1.75 hours of boiling the contents
of the 1000 ml flask and remove trap G.
Titration. - Titrate the contents with 0.01N
sodium hydroxide. Titrate with 0.01N NaOH to a yellow
end-point that persists for at least twenty seconds. Compute the
sulfite content, expressed as micrograms sulfur dioxide per gram of
food (ppm) as follows:
ppm = (32.03 × VB × N × 1000) ÷ Wt where 32.03 = milliequivalent
weight of sulfur dioxide; VB = volume of sodium hydroxide titrant
of normality, N, required to reach endpoint; the factor, 1000,
converts milliequivalents to microequivalents and Wt = weight (g)
of food sample introduced into the 1000 ml flask.
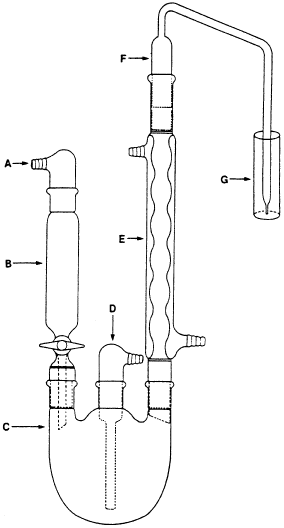
Figure 1. The
optimized Monier-Williams apparatus. Component identification is
given in text.
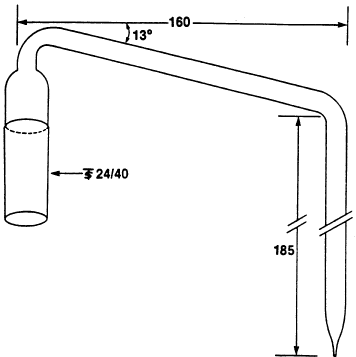
Figure 2. Diagram
of bubbler (F in Figure 1). Lengths are given in mm. [42 FR 14308,
Mar. 15, 1977, as amended at 51 FR 25017, July 9, 1986]