Title 40
SECTION 799.6756
799.6756 TSCA partition coefficient (n-octanol/water), generator column method.
§ 799.6756 TSCA partition coefficient (n-octanol/water), generator column method.(a) Scope - (1) Applicability. This section is intended to meet the testing requirements of the Toxic Substances Control Act (TSCA) (15 U.S.C. 2601).
(2) Source. The source material used in developing this TSCA test guideline is the Office of Pollution Prevention, Pesticides and Toxic Substances (OPPTS) harmonized test guideline 830.7560 (August 1996, final guideline). This source is available at the address in paragraph (e) of this section.
(b)(1) Purpose. (i) The measurement and estimation of the n-octanol/water partition coefficient (Kow), has become the cornerstone of a myriad of structure-activity relationships (SAR) property. The coefficient has been used extensively for correlating structural changes in drugs with changes observed in biological, biochemical, or toxic effects. These correlations are then used to predict the effect of a new drug for which a Kow could be measured.
(ii) In the study of the environmental fate of organic chemicals, the Kow has become a key parameter. Kow is correlated to water solubility, soil/sediment sorption coefficient, and bioconcentration and is important to SAR.
(iii) Of the three properties that can be estimated from Kow, water solubility is the most important because it affects both the fate and transport of chemicals. For example, highly soluble chemicals become quickly distributed by the hydrologic cycle, have low-sorption coefficients for soils and sediments, and tend to be more easily degraded by microorganisms. In addition, chemical transformation processes such as hydrolysis, direct photolysis, and indirect photolysis (oxidation) tend to occur more readily if a compound is soluble.
(iv) Direct correlations between Kow and both the soil/sediment sorption coefficient and the bioconcentration factor are to be expected. In these cases, compounds that are more soluble in n-octanol (more hydrophobic and lipophilic) would be expected to partition out of the water and into the organic portion of soils/sediments and into lipophilic tissue. The relationship between Kow and the bioconcentration factor, are the principal means of estimating bioconcentration factors. This relationship is discussed in the reference listed in paragraph (e)(14) of this section. These factors are then used to predict the potential for a chemical to accumulate in living tissue.
(v) This section describes a method for determining the Kow based on the dynamic coupled column liquid chromatographic (DCCLC) technique, a technique commonly referred to as the generator column method. The method described herein can be used in place of the standard shake-flask method specified in § 799.6755 for compounds with a log10Kow greater than 1.0.
(2) Definitions. The following definitions apply to this section.
Extractor column is used to extract the solute from the aqueous solution produced by the generator column. After extraction onto a bonded chromatographic support, the solute is eluted with a solvent/water mixture and subsequently analyzed by high-performance liquid chromatography (HPLC), gas chromatography (GC), or any other analytical procedure. A detailed description of the preparation of the extractor column is given in paragraph (c)(1)(i) of this section.
Generator column is used to partition the test substance between the n-octanol and water phases. The column in figure 1 in paragraph (c)(1)(i)(A)(2) of this section is packed with a solid support and is coated with the test substance at a fixed concentration in n-octanol. The test substance is eluted from the column with water and the aqueous solution leaving the column represents the equilibrium concentration of the test substance that has partitioned from the n-octanol phase into the water phase. Preparation of the generator column is described in paragraph (c)(1)(i) of this section.
n-Octanol/water partition coefficient (Kow) is defined as the ratio of the molar concentrations of a chemical in n-octanol and water, in dilute solution. The coefficient Kow is a constant for a given chemical at a given temperature. Since Kow is the ratio of two molar concentrations, it is a dimensionless quantity. Sometimes Kow is reported as the decadic logarithm (log10Kow). In this equation, Coctanol and Cwater are the molar concentration of the solute in n-octanol and water, respectively, at a given temperature. This test procedure determines Kow at 25 ±0.05 °C. The mathematical statement of Kow is:
Response factor (RF) is the solute concentration required to give a one unit area chromatographic peak or one unit output from the HPLC recording integrator at a particular recorder and detector attenuation. The factor is required to convert from units of area to units of concentration. The determination of the RF is given in paragraph (c)(3)(iii)(C)(2) of this section.
Sample loop is a 1/16 inch (in) outside diameter (O.D.) (1.6 millimeter (mm)) stainless steel tube with an internal volume between 20 and 50 µL. The loop is attached to the sample injection valve of the HPLC and is used to inject standard solutions into the mobile phase of the HPLC when determining the RF for the recording integrator. The exact volume of the loop must be determined as described in paragraph (c)(3)(iii)(C)(1) of this section when the HPLC method is used.
(3) Principle of the test method. (i) This test method is based on the DCCLC technique for determining the aqueous solubility of organic compounds. The development of this test method is described in the references listed in paragraphs (e)(6), (e)(12), and (e)(19) of this section. The DCCLC technique utilizes a generator column, extractor column, and HPLC coupled or interconnected to provide a continuous closed-flow system. Aqueous solutions of the test compound are produced by pumping water through the generator column that is packed with a solid support coated with an approximately 1.0% weight/weight (w/w) solution of the compound in n-octanol. The aqueous solution leaving the column represents the equilibrium concentration of the test chemical which has partitioned from the n-octanol phase into the water phase. The compound is extracted from the aqueous solution onto an extractor column, then eluted from the extractor column with a solvent/water mixture and subsequently analyzed by HPLC using a variable wavelength ultraviolet (UV) absorption detector operating at a suitable wavelength. Chromatogram peaks are recorded and integrated using a recording integrator. The concentration of the compound in the effluent from the generator column is determined from the mass of the compound (solute) extracted from a measured volume of water (solvent). The Kow is calculated from the ratio of the molar concentration of the solute in the 1.0% (w/w) n-octanol and molar concentration of the solute in water as determined using the generator column technique.
(ii) Since the HPLC method is only applicable to compounds that absorb in the UV, an alternate GC method, or any other reliable quantitative procedure must be used for those compounds that do not absorb in the UV. In the GC method the saturated solutions produced in the generator column are extracted using an appropriate organic solvent that is subsequently injected into the GC, or any other suitable analytical device, for analysis of the test compound.
(4) Reference chemicals. (i) Columns 2, 3, 4, and 5 of table 1 in paragraph (b)(4)(ii) of this section list the experimental values of the decadic logarithm of the n-octanol/water partition coefficient (log10Kow) at 25 °C for a number of organic chemicals as obtained from the scientific literature. These values were obtained by any one of the following experimental methods: Shake-flask; generator column; reverse-phase HPLC; or reverse-phase thin-layer chromatography, as indicated in the footnotes following each literature citation. The estimation method of Hawker and Connell as described in paragraph (e)(8) of this section, correlates log10Kow with the total surface area of the molecule and was used to estimate log10Kow for biphenyl and the chlorinated biphenyls. These estimated values are listed in column 7 of table 1 in paragraph (b)(4)(ii) of this section. Recommended values of log10Kow were obtained by critically analyzing the available experimental and estimated values and averaging the best data. These recommended values are listed in column 8 of table 1 in paragraph (b)(4)(ii) of this section.
(ii) The recommended values listed in table 1 of this section have been provided primarily so that the generator column method can be calibrated and to allow the chemical laboratory the opportunity to compare its results with these values. The testing laboratory has the option of choosing its reference chemicals, but references must be given to establish the validity of the measured values of log10Kow.
Table 1 - n-Octanol/Water Partition Coefficient at 25 °C for Some Reference Compounds
| Chemical | Experimental log10Kow | Estimated log10Kow | Recommended log10Kow | ||||
|---|---|---|---|---|---|---|---|
| Hansch and Leo 1 | Generator Column Method | Banerjee 2 | Other values | Hansch and Leo 3 | Hawker and Connell 4 | ||
| Ethyl acetate | 0.73, 0.66 | 50.68 | - | - | 0.671 | - | 170.685 |
| 1-Butanol | 0.88, 0.89, 0.32, 0.88 | 50.785 | - | - | 0.823 | - | 230.852 |
| 1-Pentanol | 1.28, 1.40 | 51,53 | - | - | 1.35 | - | 171.39 |
| Nitrobenzene | 1.85, 1.88, 1.79 | 51.85 | 1.83 | 61.82 | 1.89 | - | 171.84 |
| Benzene | 2.15, 2.13 | - | 2.12 | - | 2.14 | - | 172.14 |
| Trichloroethylene | 2.29 | 52.53 | 2.42 | - | 2.27 | - | 172.38 |
| Chlorobenzene | 2.84, 2.46 | 72.98 | - | 82.84 | 2.86 | - | 182.80 |
| o-Dichlorobenzene | 3.38 | 73.38 | 3.40 | 83.38 | 3.57 | - | 173.42 |
| n-Propylbenzene | 3.66, 3.66, 3.68, 3.57 | 53.69 | - | - | 3.85 | - | 173.69 |
| Biphenyl | 3.95, 4.17, 4.09, 4.04 | 73.67, 93.89, 103.79 | 4.04 | 63.75 | 4.03 | 4.09 | 173.96 |
| 2-Chlorobiphenyl | - | 74.50, 94.38 | - | 103.90, 113.75, 124.59, 134.54 | - | 4.99 | 194.49 |
| 1,2,3,5-Tetrachlorobenzene | - | 74.65 | 4.46 | - | 4.99 | - | 174.70 |
| 2,2′-Dichlorobiphenyl | - | 94.90 | - | 94.90, 103.63, 113.55, 144.51, 155.02 | - | 4.65 | 204.80 |
| Pentachlorobenzene | - | 75.03 | 4.94 | - | 5.71 | - | 244.99 |
| 2,4,5-Trichlorobiphenyl | - | 75.51, 95.81 | - | 105.67, 105.86, 155.77 | - | 5.60 | 175.70 |
| 2,3,4,5-Tetrachlorobiphenyl | - | 46.18, 75.72 | - | - | - | 6.04 | 175.98 |
| 2,2′,4,5,5′-Pentachlorobi-phenyl | 6.11 | 96.50, 75.92 | - | 136.11, 126.85 | - | 6.38 | 176.31 |
| 2,2′,3,3′,6,6′-Hexachloro-biphenyl | - | 45.76, 76.63, 96.81 | - | - | - | 6.22 | 176.36 |
| 2,2′,3,3′,4,4′,6-Heptachlorobiphenyl | - | 76.68 | - | - | - | 7.11 | 176.90 |
| 2,2′,3,3′,5,5′,6,6′-Octachlorobiphenyl | - | 77.11, 97.14 | - | 128.42 | - | 7.24 | 217.16 |
| 2,2′,3,3′,4, 4′,5,6,6′-Nona-chlorobiphenyl | - | 47.52 | - | - | - | 7.74 | 177.63 |
| 2,2′,3,3′,4, 5,5′6,6′-Nona-chlorobiphenyl | - | 78.16 | - | - | - | 7.71 | 177.94 |
| Decachlorobiphenyl | - | 78.26, 98.20 | - | 129.60 | - | 8.18 | 228.21 |
1 Hansch and Leo (1979). Shake-flask method in paragraph (e)(8) of this section.
2 Banerjee, Yalkowski, and Valvani (1980). Shake-flask method in paragraph (e)(1) of this section.
3 Hansch and Leo (1984). Estimates log10Kow using the CLogP3 computer program in paragraph (e)(9) of this section.
4 Hawker and Connell (1988). Generator column method and an estimation method correlating log10Kow with the total surface area of the molecule in paragraph (e)(8) of this section.
5 Tewari et al. (1982). Generator column method in paragraph (e)(14) of this section.
6 Veith, Austin, and Morris (1979). Reverse-phase HPLC method in paragraph (e)(16) of this section.
7 Miller et al. (1984). Generator column method in paragraph (e)(11) of this section.
8 Chiou and Schmedding (1982). Shake-flask method in paragraph (e)(4) of this section.
9 Woodburn, Doucette, and Andren (1984). Generator column method in paragraph (e)(19) of this section.
10 Rapaport and Eisenreich (1984). Reverse-phase HPLC method in paragraph (e)(13) of this section.
11 Woodburn (1982). Reverse-phase HPLC method in paragraph (e)(18) of this section.
12 Bruggemann, Van der Steen, and Hutzinger (1978). Shake-flask method in paragraph (e)(2) of this section.
13 Tulp and Hutzinger (1978). Shake-flask method in paragraph (e)(15) of this section.
14 Chiou, Porter, and Schmedding (1983). Shake-flask method in paragraph (e)(5) of this section.
15 Bruggemann, Van Der Steen , and Hutzinger (1982). Reverse-phase thin-layer chromatography in paragraph (e)(2) of this section.
16 Chiou et al. (1977). Shake-flask method in paragraph (e)(3) of this section.
17 Average value using all the data.
18 Average value using all the data except the datum point 2.46.
19 Average value using all the data except the data points 3.90 and 3.75.
20 Average value using all the data except the data points 3.63 and 3.55.
21 Average value using all the data except the datum point 8.42.
22 Average value using all the data except the datum point 9.60.
23 Average value using all the data except the datum point 0.32.
24 Average value using all the data excluding the estimated datum point 5.71.
(5) Applicability and specificity. The test guideline is designed to determine the Kow of solid or liquid organic chemicals in the range log10Kow 1.0 to ≤6.0 (10 to ≤10 6).
(c) Test procedure - (1) Test conditions - (i) Special laboratory equipment - (A)(1) Generator column. Either of two different methods for connecting to the generator column shall be used depending on whether the eluted aqueous phase is analyzed by HPLC (Procedure A, as described in paragraph (c)(3)(iii) of this section) or by solvent extraction followed by GC analysis, or any other reliable method of solvent extract (Procedure B, as described in paragraph (c)(3)(iv) of this section).
(2)(i) The design of the generator column is shown in the following figure 1:
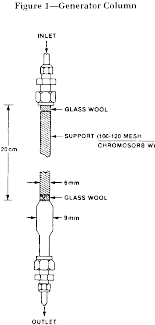
(ii) The column consists of a 6 mm ( 1/4 in) O.D. pyrex tube joined to a short enlarged section of 9 mm pyrex tubing which in turn is connected to another section of 6 mm ( 1/4 in) O.D. pyrex tubing. Connections to the inlet teflon tubing ( 1/8 in O.D.) and to the outlet stainless steel tubing ( 1/16 in O.D.) are made by means of stainless steel fittings with teflon ferrules. The column is enclosed in a water jacket for temperature control as shown in the following figure 2:
Figure 2 - Setup Showing Generator Column Enclosed in a Water Jacket and Overall Arrangement of the Apparatus Used in GC Method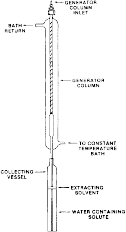
(B) Constant temperature bath with circulation pump-bath and capable of controlling temperature to 25 ±0.05 °C. (Procedures A and B, as described in paragraphs (c)(3)(iii) and (c)(3)(iv) of this section, respectively).
(C) HPLC equipped with a variable wavelength UV absorption detector operating at a suitable wavelength and a recording integrator (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(D) Extractor column - 6.6 × 0.6 centimeter (cm) stainless steel tube with end fittings containing 5 micron frits filled with a superficially porous phase packing (such as Bondapack C18 Corasil: Waters Associates) (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(E) Two 6-port high-pressure rotary switching valves (Procedure A, as described in paragraph (c)(3)(iii) of this section).
(F) Collection vessel - 8 × 3/4 in section of pyrex tubing with a flat bottom connected to a short section of 3/8 in O.D. borosilicate glass tubing. The collecting vessel is sealed with a 3/8 in teflon cap fitting (Procedure B, as described in paragraph (c)(3)(iv) of this section).
(G) GC, or any other reliable analytic equipment, equipped with a detector sensitive to the solute of interest (Procedure B, as described in paragraph (c)(3)(iv) of this section).
(ii) Purity of n-octanol and water. Purified n-octanol, described in paragraph (c)(2)(i) of this section, and water meeting appropriate American Society for Testing and Materials Type II standards, or an equivalent grade, are recommended to minimize the effects of dissolved salts and other impurities. An ASTM Type II water standard is presented in the reference listed in paragraph (e)(20) of this section).
(iii) Purity of solvents. It is important that all solvents used in this method be reagent or HPLC grade and contain no impurities which could interfere with the determination of the test compound.
(iv) Reference compounds. In order to ensure that the HPLC system is working properly, at least two of the reference compounds listed in table 1 in paragraph (b)(4)(ii) of this section should be run. Reference compounds shall be reagent or HPLC grade to avoid interference by impurities.
(2) Preparation of reagents and solutions - (i) n-Octanol and water. Very pure n-octanol can be obtained as follows: Wash pure n-octanol (minimum 98% pure) sequentially with 0.1N H2SO4, with 0.1N NaOH, then with distilled water until neutral. Dry the n-octanol with magnesium sulfate and distill twice in a good distillation column under reduced pressure [b.p. about 80 °C at 0.27 kPa (2 torr)]. The n-octanol produced should be at least 99.9% pure. Alternatively, a grade equivalent to Fisher Scientific Co. No. A-402 “Certified Octanol-1” can be used. Reagent-grade water shall be used throughout the test procedure, such as ASTM Type II water, or an equivalent grade, as described in paragraph (c)(1)(ii) of this section.
(ii) Presaturated water. Prepare presaturated water with n-octanol to minimize the depletion of n-octanol from the column when measuring the Kowof a test chemical. This is very important when the test chemical is lipophilic and the log10Kow ≤4.
(3) Performance of the test. Initially, an approximately 1.0% (w/w) solution of the test substance in n-octanol is prepared. Precise measurement of the solute concentration in this solution is required for the Kowcalculation. Subsequently, the 1.0% (w/w) solution is coated on the generator column and using either Procedure A or Procedure B as described in paragraphs (c)(3)(iii) and (c)(3)(iv) of this section, the molar concentration of the test substance in reagent-grade water is determined.
(i) Test solution. The test solution consists of an approximately 1.0% (w/w) solution of the test substance in n-octanol. A sufficient quantity (about 10-20 milliliter (mL)) of the test solution should be prepared to coat the generator column. The solution is prepared by accurately weighing out, using a tared bottle, quantities of both the test substance and n-octanol required to make a 1.0% (w/w) solution. When the weights are measured precisely (to the nearest 0.1 milligram (mg)), knowing the density of n-octanol (0.827 gram (g)/mL at 25 °C), then the molar concentration of the test substance in the n-octanol is sufficiently accurate for the purposes of the test procedure. If desired, however, a separate analytical determination (e.g., by GC, or any other reliable analytical method) may be used to check the concentration in the test solution. If storage is required, the test solution should be kept stoppered to prevent volatilization of the test chemical.
(ii) Test procedures. Prior to the determination of the Kow of the test chemical, two procedures shall be followed:
(A) The saturated aqueous solution leaving the generator column shall be tested for the presence of an emulsion, using a Tyndall procedure (i.e. light scattering). If colloids are present, they must be removed prior to injection into the extractor column by lowering the flow rate of water.
(B) The efficiency of removal of the solute (the test chemical) by solvent extraction from the extractor column shall be determined and used in the determination of the Kow of the test chemical.
(iii) Procedure A - HPLC method. (A) Procedure A covers the determination of the aqueous solubility of compounds which absorb in the UV. Two reciprocating piston pumps deliver the mobile phase (water or solvent/water mixture) through two 6-port high-pressure rotary valves and a 30 × 0.6 cm C18 analytical column to a UV absorption detector operating at a suitable wavelength. Chromatogram peaks are recorded and integrated with a recording integrator. One of the 6-port valves is the sample injection valve used for injecting samples of standard solutions of the solute in an appropriate concentration for determining RFs or standard solutions of basic chromate for determining the sample-loop volume. The other 6-port valve in the system serves as a switching valve for the extractor column which is used to remove solute from the aqueous solutions. The HPLC analytical system is shown schematically in the following figure 3:
Figure 3 - Schematic of HPLC - Generator Column Flow System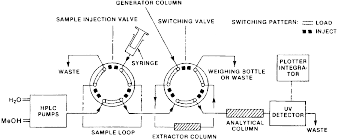
(B) The general procedure for analyzing the aqueous phase after equilibration is as follows; a detailed procedure is given in paragraph (c)(3)(iii)(C)(4) of this section:
(1) Direct the aqueous solution from the generator column to “Waste” in figure 3 in paragraph (c)(3)(iii)(A) of this section with the switching valve in the inject position in order to equilibrate internal surfaces with the solution, thus insuring that the analyzed sample would not be depleted by solute adsorption on surfaces upstream from the valve.
(2) At the same time, water is pumped from the HPLC pumps in order to displace the solvent from the extractor column.
(3) The switching valve is next changed to the load position to divert a sample of the solution from the generator column through the extractor column, and the liquid leaving the extractor column is collected in a tared weighing bottle. During this extraction step, the HPLC mobile phase is changed to a solvent/water mixture to condition the analytical column.
(4) After the desired volume of sample is extracted, the switching valve is returned to the inject position for elution from the extractor column and analysis. Assuming that all of the solute was adsorbed by the extractor column during the extraction step, the chromatographic peak represents all of the solute in the extracted sample, provided that the extraction efficiency is 100%. If the extraction efficiency is less than 100%, then the extraction efficiency shall be measured and used to determine the actual amount of the solute extracted.
(5) The solute concentration in the aqueous phase is calculated from the peak area, the weight of the extracted liquid collected in the weighing bottle, the extraction efficiency, and the RF.
(C)(1) Determination of the sample-loop volume. Accurate measurement of the sample loop may be accomplished by using a spectrophotometric method such as the one described in the reference listed in paragraph (e)(6) of this section. For this method, measure absorbance, Aloop, at 373 nanometers (nm) for at least three solutions, each of which is prepared by collecting from the sample valve an appropriate number, n, of loopfuls of an aqueous stock solution of K2CrO4 (1.3% by weight) and diluting to 50 mL with 0.2% KOH. (For a 20 µL loop, use n = 5; for a 50 µL loop, use n = 2.) Also measure the absorbance, Astock, of the same stock solution after diluting 1:500 with 0.2% KOH. Calculate the loop volume to the nearest 0.1 µL using the relation:
(2) Determination of the RF. (i) For all determinations adjust the mobile phase solvent/water ratio and flow rate to obtain a reasonable retention time on the HPLC column. For example, typical concentrations of organic solvent in the mobile phase range from 50 to 100% while flow rates range from 1 to 3 mL/minutes (min); these conditions often give a 3 to 5 min retention time.
(ii) Prepare standard solutions of known concentrations of the solute in a suitable solvent. Concentrations must give a recorder response within the maximum response of the detector. Inject samples of each standard solution into the HPLC system using the calibrated sample loop. Obtain an average peak area from at least three injections of each standard sample at a set detector absorbance unit full scale (AUFS), i.e., at the same absorbance scale attenuation setting.
(iii) Calculate the RF from the following equation:
(3) Loading of the generator column. (i) The design of the generator column was described in paragraph (c)(1)(i) of this section and is shown in figure 1 in paragraph (c)(1)(i)(A)(2)(i) of this section. To pack the column, a plug of silanized glass wool is inserted into one end of the 6 mm pyrex tubing. Silanized diatomaceous silica support (about 0.5g of 100-120 mesh Chromosorb W chromatographic support material) is poured into the tube with tapping and retained with a second plug of silanized glass wool.
(ii) The column is loaded by pulling the test solution through the dry support with gentle suction and then allowing the excess solution to drain out. After loading the column, draw water up through the column to remove any entrapped air.
(4) Analysis of the solute. Use the following procedure to collect and analyze the solute:
(i) With the switching valve in figure 3 in paragraph (c)(3)(iii)(A) of this section in the inject position (i.e., water to waste), pump water through the generator column at a flow rate of approximately 1 mL/min for approximately 15 min to bring the system into equilibrium. Pump water to the generator column by means of a minipump or pressurized water reservoir as shown in the following figure 4:
Figure 4 - Water Reservoir for GC Method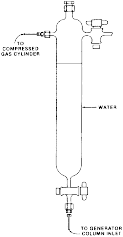
(ii) Flush out the organic solvent that remains in the system from previous runs by changing the mobile phase to 100% H2O and allowing the water to reach the HPLC detector, as indicated by a negative reading. As soon as this occurs, place a 25 mL weighing bottle (weighed to the nearest mg) at the waste position and immediately turn the switching valve to the load position.
(iii) Collect an amount of water from the generator column (as determined by trial and error) in the weighing bottle, corresponding to the amount of solute adsorbed by the extractor column that gives a reasonable detector response. During this extraction step, switch back to the original HPLC mobile phase composition, i.e., solvent/water mixture, to condition the HPLC analytical column.
(iv) After the desired volume of sample has been extracted, turn the switching valve back to the inject position in figure 3 in paragraph (c)(3)(iii)(A) of this section. As soon as the switching valve is turned to the inject position, remove the weighing bottle, cap it and replace it with the waste container; at the same time turn on the recording integrator. The solvent/water mobile phase will elute the solute from the extractor column and transfer the solute to the HPLC analytical column.
(v) Determine the weight of water collected to the nearest mg and record the corresponding peak area. Using the same AUFS setting repeat the analysis of the solute at least two more times and determine the average ratio of peak area to grams of water collected. In this equation, S = solubility (M), RF = response factor, Vloop = sample-loop volume (L), and R = ratio of area to grams of water. Calculate the solute solubility in water using the following equation:
(iv) Procedure B - GC Method. In the GC method, or any other reliable quantitative method, aqueous solutions from the generator column enter a collecting vessel in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section containing a known weight of extracting solvent which is immiscible in water. The outlet of the generator column is positioned such that the aqueous phase always enters below the extracting solvent. After the aqueous phase is collected, the collecting vessel is stoppered and the quantity of aqueous phase is determined by weighing. The solvent and the aqueous phase are equilibrated by slowly rotating the collecting vessel. A small amount of the extracting solvent is then removed and injected into a GC equipped with an appropriate detector. The solute concentration in the aqueous phase is determined from a calibration curve constructed using known concentrations of the solute. The extraction efficiency of the solvent shall be determined in a separate set of experiments.
(A) Determination of calibration curve. (1) Prepare solute standard solutions of concentrations covering the expected range of the solute solubility. Select a column and optimum GC operating conditions for resolution between the solute and solvent and the solute and extracting solvent. Inject a known volume of each standard solution into the injection port of the GC. For each standard solution determine the average of the ratio R of peak area to volume (in µL) for the chromatographic peak of interest from at least three separate injections.
(2) After running all the standard solutions, determine the coefficients, a and b, using linear regression analysis on the equation of concentration (C) vs. R in the form:
(B) Loading of the generator column. The generator column is packed and loaded with solute in the same manner as for the HPLC method in paragraph (c)(3)(iii) of this section. As shown in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section, attach approximately 20 cm of straight stainless steel tubing to the bottom of the generator column. Connect the top of the generator column to a water reservoir in figure 4 in paragraph (c)(3)(iii)(C)(4)(i) of this section using teflon tubing. Use air or nitrogen pressure (5 PSI) from an air or nitrogen cylinder to force water from the reservoir through the column. Collect water in an Erlenmeyer flask for approximately 15 min while the solute concentration in water equilibrates; longer time may be required for less soluble compounds.
(C) Collection and extraction of the solute. During the equilibration time, add a known weight of extracting solvent to a collection vessel which can be capped. The extracting solvent should cover the bottom of the collection vessel to a depth sufficient to submerge the collecting tube but still maintain 100:1 water/solvent ratio. Record the weight (to the nearest mg) of a collection vessel with cap and extracting solvent. Place the collection vessel under the generator column so that water from the collecting tube enters below the level of the extracting solvent in figure 2 in paragraph (c)(1)(i)(A)(2)(ii) of this section. When the collection vessel is filled, remove it from under the generator column, replace cap, and weigh the filled vessel. Determine the weight of water collected. Before analyzing for the solute, gently rotate the collection vessel contents for approximately 30 min, controlling the rate of rotation so as not to form an emulsion; rotating the flask end over end five times per minute is sufficient. The extraction efficiency of the solvent shall be determined in a separate set of experiments.
(D) Analysis of the solute. (1) After rotating, allow the collection vessel to stand for approximately 30 min; then remove a known volume of the extracting solvent from the vessel using a microliter syringe and inject it into the GC. Record the ratio of peak area to volume injected and, from the regression equation of the calibration line, determine the concentration of solute in the extracting solvent. If the extraction efficiency is not 100%, the measured extraction efficiency shall be used to obtain the correct concentration of solute extracted. In this equation, Ces is the molar concentration of solute in extracting solvent, dH2O and des are the densities in grams per milliliter of water and extracting solvent, respectively, and ges and gH2O are the grams of extracting solvent and water, respectively, contained in the collection vessels. The molar concentration of solute in water C(M) is determined from the following equation:
(2) Make replicate injections from each collecting vessel to determine the average solute concentration in water for each vessel. To make sure the generator column has reached equilibrium, run at least two additional (for a total of three) collection vessels and analyze the extracted solute as described in paragraph (c)(3)(iv)(D)(1) of this section. Calculate C(M) from the average solute concentration in the three vessels.
(3) If another analytical method is used in place of the GC, then Procedure B, as described in paragraph (c)(3)(iv) of this section, shall be modified and the new analytical procedure shall be used to determine quantitatively the amount of solute extracted in the extraction solvent.
(v) Analysis of reference compounds. Prior to analyzing the test solution, make duplicate runs on at least two of the reference compounds listed in table 1 in paragraph (b)(4)(ii) of this section. When using the reference compounds, follow the same procedure previously described for preparing the test solution and running the test. If the average value obtained for each compound is within 0.1 log unit of the reference value, then the test procedure and HPLC system are functioning properly; if not a thorough checking over of the HPLC and careful adherence to the test procedures should be done to correct the discrepancy.
(vi) Modification of procedures for potential problems - Decomposition of the test compound. If the test compound decomposes in one or more of the aqueous solvents required during the period of the test at a rate such that an accurate value for water solubility cannot be obtained, then it will be necessary to carry out detailed transformation studies, such as hydrolysis studies. If decomposition is due to aqueous photolysis, then it will be necessary to carry out the studies in the dark, under red or yellow lights, or by any other suitable method to eliminate this transformation process.
(d) Data and reporting - (1) Test report. (i) For the test solution, report the weights to the nearest 0.1 mg of the test substance and n-octanol. Also report the weight percent and molar concentration of the test substance in the n-octanol; the density of n-octanol at 25 °C is 0.827 grams per milliliter (gm)/mL.
(ii) For each run provide the molar concentration of the test substance in water for each of three determinations, the mean value, and the standard deviation.
(iii) For each of the three determinations calculate the Kow as the ratio of the molar concentration of the test substance in n-octanol to the molar concentration in water. Also calculate and report the mean Kow and its standard deviation. Values of Kow shall be reported as their logarithms (log10Kow).
(iv) Report the temperature (±0.05 °C) at which the generator column was controlled during the test.
(v) For each reference compound report the individual values of log10Kow and the average of the two runs.
(vi) For compounds that decompose at a rate such that a precise value for the solubility cannot be obtained, provide a statement to that effect.
(2) Specific analytical, calibration, and recovery procedures. (i) For the HPLC method describe and/or report:
(A) The method used to determine the sample-loop volume and the average and standard deviation of that volume.
(B) The average and standard deviation of the RF.
(C) The extraction solvent and the extraction efficiency used.
(D) Any changes made or problems encountered in the test procedures.
(ii) For the GC method report:
(A) The column and GC operating conditions of temperature and flow rate.
(B) The average and standard deviation of the average area per microliter obtained for each of the standard solutions.
(C) The form of the regression equation obtained in the calibration procedure.
(D) The extracting solvent and extraction efficiency used.
(E) The average and standard deviation of solute concentration in each collection vessel.
(F) Any changes made or problems encountered in the test procedure.
(iii) If another approved analytical method is used to determine the concentration of the test chemical in water, then all the important test conditions shall be reported.
(iv) If the concentration of the test substance in n-octanol is determined by an independent analytical method such as GC, provide a complete description of the method.
(e) References. For additional background information on this test guideline, the following references should be consulted. These references are available at the addresses in § 700.17(b)(1) and (2) of this chapter.
(1) Banerjee, S. et al., Water solubility and octanol/water partition coefficient of organics. Limitation of the solubility-partition coefficient correlation. Environmental Science and Technology 14:1227-1229 (1980).
(2) Bruggemann W.A. et al., Reversed-phase thin-layer chromatography of polynuclear aromatic hydrocarbons and chlorinated biphenyls. Relationship with hydrophobicity as measured by aqueous solubility and octanol/water partition coefficient. Journal of Chromatography 238: 335-346 (1982).
(3) Chiou, C.T. et al. Partition coefficient and bioaccumulation of selected organic chemicals. Environmental Science and Technology 11:475-478 (1977).
(4) Chiou, C.T. and Schmedding, D.W., Partitioning of organic compounds in octanol/water systems. Environmental Science and Technology 16:4-10 (1982).
(5) Chiou, C.T et al., Partition equilibria of nonionic organic compounds between soil, organic matter, and water. Environmental Science and Technology 17:227-231 (1983).
(6) DeVoe, H. et al. “Generator Columns and High Pressure Liquid Chromatography for Determining Aqueous Solubilities and Octanol-Water Partition Coefficients of Hydrophobic Substances,” Journal of Research of the National Bureau of Standards, 86:361-366 (1981).
(7) Fujita, T. et al. “A New Substituent Constant, Derived from Partition Coefficients.” Journal of the American Chemical Society, 86:5175 (1964).
(8) Hansch, C. and Leo, A. 1985 MEDCHEM Project, version 26. Pomona College, Claremont, CA. USA.
(9) Hansch, C. and Leo, A. Medchem Software Manual. CLOGP3 Users Guide. Release 3.32. December 1984. Medicinal Chemistry Project, Pomona College, Claremont, CA.
(10) Hawker, D.W. and Connell, D.W. Octanol-water partition coefficients of polychlorinated biphenyl congeners. Environmental Science and Technology 22:382-387 (1988).
(11) May, W.E. et al. “Determination of the aqueous solubility of polynuclear aromatic hydrocarbons by a coupled column liquid chromatographic technique,” Analytical Chemistry, 50:175-179 (1978).
(12) May, W.E. et al. “Determination of the Solubility Behavior of Some Polycyclic Aromatic Hydrocarbons in Water,” Analytical Chemistry 50:997-1000 (1978).
(13) Miller, M.M. et al. Aqueous solubilities, octanol/water partition coefficients and entropies of melting of chlorinated benzenes and biphenyls. Journal of Chemical and Engineering Data 29:184-190 (1984).
(14) Neely, W.B. et al. Partition Coefficient to Measure Bioconcentration Potential of Organic Chemicals in Fish, Environmental Science Technology, 8:113-115 (1974).
(15) Rappaport, R.A. and Eisenrich, S.J. Chromatographic determination of octanol-water partition coefficients (Kow's) for 58 polychlorinated biphenyl congeners. Environmental Science and Technology 18:163-170 (1984).
(16) Tewari, Y.B. et al. Aqueous solubility and octanol/water partition coefficients of organic compounds at 25 °C. Journal of Chemical and Engineering Data 27:451-454 (1982).
(17) Tulp, M.T.M. and Hutzinger, O. Some thoughts on aqueous solubilities and partition coefficients of PCB, and the mathematical correlation between bioaccumulation and physio-chemical properties. Chemosphere 10:849-860 (1978).
(18) Veith, G.D. et al. A rapid method for estimating log10 P for organic chemicals, Water Research 13:43-47 (1979).
(19) Wasik, S.P. et al. Octanol/water partition coefficient and aqueous solubilities of organic compounds, Report NBSIR 81-2406 (1981). National Bureau of Standards, U.S. Department of Commerce, Washington, DC.
(20) Woodburn, K.B. Measurement and application of the octanol/water partition coefficients for selected polychlorinated biphenyls. Master's Thesis (1982), University of Wisconsin at Madison, Madison, WI.
(21) Woodburn, K.B. et al. Generator column determination of octanol/water partition coefficients for selected polychlorinated biphenyl congeners. Environmental Science and Technology 18:457-459 (1984).
(22) ASTM D 1193-91 (Approved Sep 15, 1991), “Standard Specification for Reagent Water.” American Society for Testing and Materials (ASTM), 1916 Race St., Philadelphia, PA 19103.
[65 FR 78751, Dec. 15, 2000, as amended at 77 FR 46293, Aug. 3, 2012]