Title 40
SECTION 796.3100
796.3100 Aerobic aquatic biodegradation.
§ 796.3100 Aerobic aquatic biodegradation.(a) Introduction - (1) Purpose. (i) This Guideline is designed to develop data on the rate and extent of aerobic biodegradation that might occur when chemical substances are released to aquatic environments. A high biodegradability result in this test provides evidence that the test substance will be biodegradable in natural aerobic freshwater environments.
(ii) On the contrary, a low biodegradation result may have other causes than poor biodegradability of the test substance. Inhibition of the microbial inoculum by the test substance at the test concentration may be observed. In such cases, further work is needed to assess the aerobic aquatic biodegradability and to determine the concentrations at which toxic effects are evident. An estimate of the expected environmental concentration will help to put toxic effects into perspective.
(2) Definitions. (i) “Adaptation” is the process by which a substance induces the synthesis of any degradative enzymes necessary to catalyze the transformation of that substance.
(ii) “Ultimate Biodegradability” is the breakdown of an organic compound to CO2, water, the oxides or mineral salts of other elements and/or to products associated with normal metabolic processes of microorganisms.
(iii) “Ready Biodegradability” is an expression used to describe those substances which, in certain biodegradation test procedures, produce positive results that are unequivocal and which lead to the reasonable assumption that the substance will undergo rapid and ultimate biodegradation in aerobic aquatic environments.
(3) Principle of the test method. This Guideline method is based on the method described by William Gledhill (1975) under paragraph (d)(1) of this section. The method consists of a 2-week inoculum buildup period during which soil and sewage microorganisms are provided the opportunity to adapt to the test compound. This inoculum is added to a specially equipped Erlenmeyer flask containing a defined medium with test substance. A reservoir holding barium hydroxide solution is suspended in the test flask. After inoculation, the test flasks are sparged with CO2-free air, sealed, and incubated, with shaking in the dark. Periodically, samples of the test mixture containing water-soluble test substances are analyzed for dissolved organic carbon (DOC) and the Ba(OH)2 from the reservoirs is titrated to measure the amount of CO2 evolved. Differences in the extent of DOC disappearance and CO2 evolution between control flasks containing no test substance, and flasks containing test substance are used to estimate the degree of ultimate biodegradation.
(4) Prerequisites. The total organic carbon (TOC) content of the test substance shall be calculated or, if this is not possible, analyzed, to enable the percent of theoretical yield of carbon dioxide and percent of DOC loss to be calculated.
(5) Guideline information. (i) Information on the relative proportions of the major components of the test substance will be useful in interpreting the results obtained, particularly in those cases where the result lies close to a “pass level.”
(ii) Information on the toxicity of the chemical may be useful in the interpretation of low results and in the selection of appropriate test concentrations.
(6) Reference substances. Where investigating a chemical substance, reference compounds may be useful and an inventory of suitable reference compounds needs to be identified. In order to check the activity of the inoculum the use of a reference compound is desirable. Aniline, sodium citrate, dextrose, phthalic acid and trimellitic acid will exhibit ultimate biodegradation under the conditions of this Test Guideline method. These reference substances must yield 60 percent of theoretical maximum CO2 and show a removal of 70 percent DOC within 28 days. Otherwise the test is regarded as invalid and shall be repeated using an inoculum from a different source.
(7) Reproducibility. The reproducibility of the method has not yet been determined; however it is believed to be appropriate for a screening test which has solely an acceptance but no rejective function.
(8) Sensitivity. The sensitivity of the method is determined by the ability to measure the endogenous CO2 production of the inoculum in the blank flask and by the sensitivity limit of the dissolved organic carbon analysis. If the test is adapted to handle 14C-labeled test substances, test substance concentrations can be much lower.
(9) Possibility of standardization. This possibility exists. The major difficulty is to standardize the inoculum in such a way that interlaboratory reproducibility is ensured.
(10) Possibility of automation. None at present, although parts of the analyses may be automated.
(b) Test procedures - (1) Preparations - (i) Apparatus. The shake flask apparatus under the following Figure 1 contains 10 mL of 0.2N Ba(OH)2 in an open container suspended over 1 liter of culture medium in a 2-liter Erlenmeyer flask.
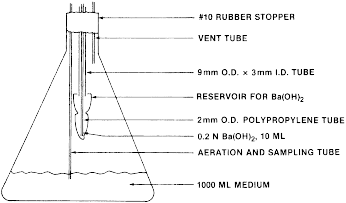 Figure 1
- Shake-Flask System for Carbon Dioxide Evolution The Ba(OH)2
container is made by placing a constriction just above the 10 mL
mark of a 50 mL heavy-duty centrifuge tube and attaching the
centrifuge tube to a 2 mm I.D. × 9 mm O.D. glass tube by means of 3
glass support rods. The centrifuge tube opening is large enough to
permit CO2 to diffuse into the Ba(OH)2, while the constriction
permits transferal of the flask to and from the shaker without
Ba(OH)2 spillage into the medium. For periodic removal and addition
of base from the center well, a polypropylene capillary tube,
attached at one end to a 10 ml disposable syringe, is inserted
through the 9 mm O.D. glass tube into the Ba(OH)2 reservoir. The
reservoir access port is easily sealed during incubation with a
serum bottle stopper. Two glass tubes are added for sparging,
venting, and medium sampling. The tops of these tubes are connected
with a short section of flexible tubing during incubation.
Figure 1
- Shake-Flask System for Carbon Dioxide Evolution The Ba(OH)2
container is made by placing a constriction just above the 10 mL
mark of a 50 mL heavy-duty centrifuge tube and attaching the
centrifuge tube to a 2 mm I.D. × 9 mm O.D. glass tube by means of 3
glass support rods. The centrifuge tube opening is large enough to
permit CO2 to diffuse into the Ba(OH)2, while the constriction
permits transferal of the flask to and from the shaker without
Ba(OH)2 spillage into the medium. For periodic removal and addition
of base from the center well, a polypropylene capillary tube,
attached at one end to a 10 ml disposable syringe, is inserted
through the 9 mm O.D. glass tube into the Ba(OH)2 reservoir. The
reservoir access port is easily sealed during incubation with a
serum bottle stopper. Two glass tubes are added for sparging,
venting, and medium sampling. The tops of these tubes are connected
with a short section of flexible tubing during incubation.
(ii) Reagents and stock solutions. (A) Stock solutions, I, II, and III under the following Table 1.
(B) Yeast extract.
(C) Vitamin-free casamino acids.
(D) 70 percent O2 in nitrogen or CO2-free air.
(E) 0.2N Ba(OH)2.
(F) 0.1 N HCl.
(G) 20 percent H2SO4.
(H) Phenolphthalein.
(I) Dilution water - distilled, deionized water (DIW).
(iii) Soil inoculum. A fresh sample of an organically rich soil is used as the inoculum in the ultimate biodegradation test. Soil is collected, prepared, and stored according to the recommendations of Pramer and Bartha (1972) under paragraph (d)(2) of this section. The soil surface is cleared of litter and a soil sample is obtained 10 to 20 cm below the surface. The sample is screened through a sieve with 2 to 5 mm openings and stored in a polyethylene bag at 2 to 4 °C for not more than 30 days prior to use. The soil is never allowed to air-dry, and shall not be frozen during storage.
Table 1 - Medium Employed for Assay of CO2 Evolution
| Solution 1 | Compound | Stock Solution Conc. (g/L) |
|---|---|---|
| I | NH4Cl | 35 |
| KNO3 | 15 | |
| K2HPO4·3H2O | 750 | |
| NaH2PO4·H2O | 25 | |
| II 2 | KCl | 10 |
| MgSO4 | 20 | |
| FeSO4·7H2O | 1 | |
| III | CaCl2 | 5 |
| ZnCl2 | 0.05 | |
| MnCl2·4H2O | 0.5 | |
| CuCl2 | 0.05 | |
| CoCl2 | 0.001 | |
| H3 BO3 | 0.001 | |
| MoO3 | 0.0004 |
1 = Each liter of test medium contains 1 mL of each solution.
2 = Final pH is adjusted to 3.0 with 0.10 N HCl.
(iv) Acclimation Medium. Acclimation medium is prepared by adding, for each liter of distilled, deionized water (DIW): 1 mL each of solutions I, II, and III in Table 1 in paragraph (b)(1)(iii) of this section, 1.0 gm of soil inoculum (prepared according to paragraph (b)(1)(iii) of this section), 2.0 mL of aerated mixed liquor (obtained from an activated sludge treatment plant not more than 2 days prior to commencing the acclimation phase, and stored in the interim at 4 °C) and 50 mL raw domestic influent sewage. This medium is mixed for 15 minutes and filtered through a glass wool plug in a glass funnel. The filtrate is permitted to stand for 1 hour, refiltered through glass wool, and supplemented with 25 mg/L each of Difco vitamin-free casamino acids and yeast extract. Appropriate volumes are added to 2-liter Erlenmeyer flasks. Test compounds are added incrementally during the acclimation period at concentrations equivalent to 4, 8, and 8 mg/L carbon on days 0, 7, and 11, respectively. On day 14, the medium is refiltered through glass wool prior to use in the test. For evaluating the biodegradability of a series of functionally or structurally related chemicals, media from all inoculum flasks may be combined before final filtration.
(2) Procedures. (i) Inoculum (100 mL of acclimation medium) is added to 900 mL DIW containing 1 mL each of solutions I, II, and III in Table 1 under paragraph (b)(1)(iii) of this section in a 2-liter Erlenmeyer flask. Test compound equivalent to 10 mg/liter carbon is added to each of the replicate flasks containing the test medium. Ten mL of 0.2 N Ba (OH)2 are added to the suspended reservoir in each flask and duplicate 10 mL samples of Ba(OH)2 are also saved as titration blanks for analysis with test samples. Flasks are sparged with CO2-free air (for volatile test materials, sparging is done prior to addition of the chemical), sealed, and placed on a gyrotary shaker (approximately 125 rpm) at 20 to 25 °C in the dark. For each set of experiments, each test, reference, inhibited, and control system should be analyzed at time zero and at a minimum of four other times from time zero through day 28. Sampling must be made with sufficient frequency to allow for a smooth plot of biodegradation with time. Sampling times should be varied by the investigator as deemed appropriate to match the rate of degradation of the test substance. Tests may be terminated when biodegradation reaches a plateau and is consistent (±10 percent) over 3 consecutive days or on day 28, whichever occurs first. For chemicals which are water soluble at the test concentration, an adequate volume (5 to 10 mL) of medium is removed for DOC analysis. Each sample for DOC analysis should be filtered through a membrane filter of 0.45 micrometer pore diameter before DOC analysis. For all test and reference compounds, Ba(OH)2 from the center well is removed for analysis. The center well is rinsed with 10 mL CO2-free DIW and is refilled with fresh base. Rinse water is combined with the Ba(OH)2 sample to be analyzed. Flasks are resealed and placed on the shaker. On the day prior to terminating the test, 3 mL of 20 percent H2SO4 are added to the medium to release carbonate bound CO2.
(ii) For each set of experiments, each test substance shall be tested in triplicate.
(iii) For each set of experiments, one or two reference compounds are included to assess the microbial activity of the test medium. Duplicate reference flasks are prepared by adding reference compound equivalent to 10 mg/liter carbon to each of two flasks containing the test medium. Reference compounds which are positive for ultimate biodegradability include: sodium citrate, dextrose, phthalic acid, trimellitic acid, and aniline.
(iv) For each test set, triplicate controls receiving inoculated medium and no test compound, plus all test and reference flasks, are analyzed for CO2 evolution and DOC removal. Results from analysis of the control flasks (DOC, CO2 evolution, etc.) are subtracted from corresponding experimental flasks containing test compound in order to arrive at the net effect due to the test compound.
(v) A test system containing a growth inhibitor should be established as a control for each substance tested for biodegradation by this method. That inhibited system must contain the same amount of water, mineral nutrients, inoculum, and test substance used in the uninhibited test systems, plus 50 mg/L mercuric chloride (HgCl2) to inhibit microbial activity.
(vi) Flasks shall be incubated in the dark to minimize both photochemical reactions and algal growth. Appropriate sterile controls or controls containing a metabolic inhibitor, such as 50 mg/1 HgCl2, are needed to correct for interferences due to nonbiological degradation. With volatile organic materials, sparging with CO2-free air is performed only once, just prior to addition of the test chemical. Analyses for CO2 evolution and DOC removal are conducted within 2 to 3 hours of sampling to minimize interferences which may occur in storage. All glassware should be free of organic carbon contaminants.
(3) Analytical measurements. The quantity of CO2 evolved is measured by titration of the entire Ba(OH)2 sample (10 mL Ba(OH)2 + 10 mL rinse water) with 0.1 N HCl to the phenolphthalein end point. Ba(OH)2 blanks are also supplemented with 10 mL CO2-free DIW and titrated in a similar manner. Samples (5 mL) for DOC are centrifuged and/or filtered and supernatant or filtrate analyzed by a suitable total organic carbon method.
(c) Data and reporting - (1) Treatment of results. (i) Test compound (10 mg carbon) is theoretically converted to 0.833 mmol CO2.−1. Absorbed CO2 precipitates as BaCO3 from Ba(OH)2, causing a reduction in alkalinity by the equivalent of 16.67 mL of 0.1 N HCl for complete conversion of the test compound carbon to CO2. Therefore, the percent theoretical CO2 evolved from the test compound is calculated at any sampling time from the formula:
Percent CO2 evolution=[(TF−CF)/16.67] 100 (for 10 mg/L test compound carbon) where: TF = mL 0.1 N HCl required to titrate Ba(OH)2 samples from the test flask CF = mL 0.1 N HCl required to titrate Ba(OH)2 samples from the control flask.(ii) The cumulative percent CO2 evolution at any sample time is calculated as the summation of the percent CO2 evolved at all sample points of the test.
(iii) The percent DOC disappearance from the test compound is calculated from the following equation:
Percent DOC Removal=[1−(DTFx− DCFx)/(DTFo− DCFo)] 100 where: DTF= Dissolved organic carbon from test flask DCF= Dissolved organic carbon from control flask o= Day zero measurements x= Day of measurements during test.(iv) The difference between the amount of 0.1 N HCl used for the Ba(OH)2 titration blank samples and the Ba(OH)2 samples from the control units (no test compound) is an indication of the activity of the microorganisms in the test system. In general, this difference is approximately 1 to 3 mL of 0.1 N HCl at each sampling time. A finding of no difference in the titration volumes between these two samples indicates a poor inoculum. In this case, the validity of the test results is questionable and the test set shall be rerun beginning with the acclimation phase.
(v) CO2 evolution in the reference flasks is also indicative of the activity of the microbial test system. The suggested reference compounds should all yield final CO2 evolution values of at least 60 percent of theoretical CO2. If, for any test set, the percent theoretical CO2 evolution value for the reference flasks is outside this range, the test results are considered invalid and the test is rerun.
(vi) Inhibition by the test compound is indicated by lower CO2 evolution in the test flasks than in the control flasks. If inhibition is noted, the study for this compound is rerun beginning with the acclimation phase. During the test phase for inhibitory compounds, the test chemical is added incrementally according to the schedule: Day 0 - 0.5 mg/liter as organic carbon, Day 2 - 1 mg/liter C, Day 4 - 1.5 mg/liter C, Day 7 - 2 mg/liter C, Day 10 - 5 mg/liter C. For this case, the Ba(OH)2 is sampled on Day 10, and weekly thereafter. The total test duration remains 28 days.
(vii) The use of 14C-labeled chemicals is not required. If appropriately labeled test substance is readily available and if the investigator chooses to use this procedure with labeled test substance, this is an acceptable alternative. If this option is chosen, the investigator may use lower test substance concentrations if those concentrations are more representative of environmental levels.
(2) Test report. (i) For each test and reference compound, the following data shall be reported.
(ii) Information on the inoculum, including source, collection date, handling, storage and adaptation possibilities (i.e., that the inoculum might have been exposed to the test substance either before or after collection and prior to use in the test).
(iii) Results from each test, reference, inhibited (with HgCl2) and control system at each sampling time, including an average result for the triplicate test substance systems and the standard deviation for that average.
(iv) Average cumulative percent theoretical CO2 evolution over the test duration.
(v) Dissolved organic carbon due to test compound at each sampling time (DTF-DCF).
(vi) Average percent DOC removal at each sampling time.
(vii) Twenty-eight day standard deviation for percent CO2 evolution and DOC removal.
(d) References. For additional background information on this test guideline the following references should be consulted:
(1) Gledhill, W.E. “Screening Test for Assessment of Ultimate Biodegradability: Linear Alkyl Benzene Sulfonate,” Applied Microbiology, 30:922-929 (1975).
(2) Pramer, D., Bartha, R. “Preparation and Processing of Soil Samples for Biodegradation Testing,” Environmental Letters, 2:217-224 (1972).
[50 FR 39252, Sept. 27, 1985, as amended at 52 FR 19058, May 20, 1987]