Title 40
SECTION 63.11950
63.11950 What emissions calculations must I use for an emission profile
§ 63.11950 What emissions calculations must I use for an emission profile?When developing your emission profiles for batch process vents as required in § 63.11925(g), except as specified in paragraph (i) of this section, you must calculate emissions from episodes caused by vapor displacement, purging a partially filled vessel, heating, depressurization, vacuum operations, gas evolution, air drying, or empty vessel purging, using the applicable procedures in paragraphs (a) through (h) of this section.
(a) Vapor displacement. You must calculate emissions from vapor displacement due to transfer of material using Equation 1 of this section.
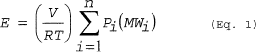
(Eq. 1)
Where: E = Mass of HAP emitted. V = Volume of gas displaced from the vessel. R = Ideal gas law constant. T = Temperature of the vessel vapor space; absolute. Pi = Partial pressure of the individual HAP. MWi = Molecular weight of the individual HAP. n = Number of HAP compounds in the emission stream. i = Identifier for a HAP compound.(b) Gas sweep of a partially filled vessel. You must calculate emissions from purging a partially filled vessel using Equation 2 of this section. The pressure of the vessel vapor space may be set equal to 760 millimeters of mercury (mmHg). You must multiply the HAP partial pressure in Equation 2 of this section by a HAP-specific saturation factor determined in accordance with Equations 3 through 5 of this section. Solve Equation 3 of this section iteratively beginning with saturation factors (in the right-hand side of the equation) of 1.0 for each condensable compound. Stop iterating when the calculated saturation factors for all compounds are the same to two significant figures for subsequent iterations. Note that for multi-component emission streams, saturation factors must be calculated for all condensable compounds, not just the HAP.
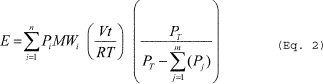
(Eq. 2)
Where: E = Mass of HAP emitted. V = Purge flow rate of the noncondensable gas at the temperature and pressure of the vessel vapor space. R = Ideal gas law constant. T = Temperature of the vessel vapor space; absolute. Pi = Partial pressure of the individual HAP at saturated conditions. Pj = Partial pressure of individual condensable compounds (including HAP) at saturated conditions. PT = Pressure of the vessel vapor space. MWi = Molecular weight of the individual HAP. t = Time of purge. n = Number of HAP compounds in the emission stream. i = Identifier for a HAP compound. j = Identifier for a condensable compound. m = Number of condensable compounds (including HAP) in the emission stream.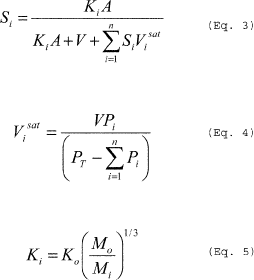 Where: Si = Saturation factor for individual condensable
compounds. Pi = Partial pressure of individual condensable
compounds at saturated conditions. PT = Pressure of the vessel
vapor space. A = Surface area of liquid. V = Purge flow rate of the
noncondensable gas. Vi sat = Volumetric flow rate of individual
condensable compounds at saturated vapor pressure. Ki = Mass
transfer coefficient of individual condensable compounds in the
emission stream. Ko = Mass transfer coefficient of reference
compound (e.g., 0.83 cm/s for water). Mo = Molecular weight
of reference compound (e.g., 18.02 for water). Mi =
Molecular weight of individual condensable compounds in the
emission stream. n = Number of condensable compounds in the
emission stream.
Where: Si = Saturation factor for individual condensable
compounds. Pi = Partial pressure of individual condensable
compounds at saturated conditions. PT = Pressure of the vessel
vapor space. A = Surface area of liquid. V = Purge flow rate of the
noncondensable gas. Vi sat = Volumetric flow rate of individual
condensable compounds at saturated vapor pressure. Ki = Mass
transfer coefficient of individual condensable compounds in the
emission stream. Ko = Mass transfer coefficient of reference
compound (e.g., 0.83 cm/s for water). Mo = Molecular weight
of reference compound (e.g., 18.02 for water). Mi =
Molecular weight of individual condensable compounds in the
emission stream. n = Number of condensable compounds in the
emission stream.
(c) Heating. You must calculate emissions caused by the heating of a vessel to a temperature lower than the boiling point using the procedures in paragraph (c)(1) of this section. If the contents of a vessel are heated to the boiling point, you must calculate emissions using the procedures in paragraph (c)(2) of this section.
(1) If the final temperature to which the vessel contents are heated is lower than the boiling point of the HAP in the vessel, you must calculate the mass of HAP emitted per episode using Equation 6 of this section. The average gas space molar volume during the heating process is calculated using Equation 7 of this section. The difference in the number of moles of condensable in the vessel headspace between the initial and final temperatures is calculated using Equation 8 of this section.
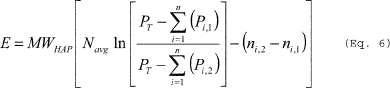
(Eq. 6)
Where: E = Mass of HAP vapor displaced from the vessel being heated. Navg = Average gas space molar volume during the heating process. PT = Total pressure in the vessel. Pi,1 = Partial pressure of the individual HAP compounds at initial temperature (T1). Pi,2 = Partial pressure of the individual HAP compounds at final temperature (T2). MWHAP = Average molecular weight of the HAP compounds calculated using Equation 13 of this section. ni,1 = Number of moles of condensable in the vessel headspace at initial temperature (T1). ni,2 = Number of moles of condensable in the vessel headspace at final temperature (T2). n = Number of HAP compounds in the emission stream. ln = Natural logarithm.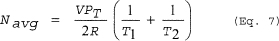
(Eq. 7)
Where: Navg = Average gas space molar volume during the heating process. V = Volume of free space in vessel. PT = Total pressure in the vessel. R = Ideal gas law constant. T1 = Initial temperature of the vessel. T2 = Final temperature of the vessel.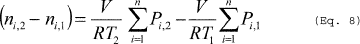 Where: V = Volume of free space in vessel. R = Ideal gas law
constant. T1 = Initial temperature in the vessel. T2 = Final
temperature in the vessel. Pi,1 = Partial pressure of the
individual HAP compounds at T1. Pi,2 = Partial pressure of the
individual HAP compounds at T2. n = Number of HAP compounds in the
emission stream.
Where: V = Volume of free space in vessel. R = Ideal gas law
constant. T1 = Initial temperature in the vessel. T2 = Final
temperature in the vessel. Pi,1 = Partial pressure of the
individual HAP compounds at T1. Pi,2 = Partial pressure of the
individual HAP compounds at T2. n = Number of HAP compounds in the
emission stream.
(2) If the final temperature to which the vessel contents are heated is at the boiling point or higher, you must calculate emissions using the procedure in paragraphs (c)(2)(i) and (ii) of this section.
(i) To calculate the emissions from heating to the boiling point use Equations 9, 10 and 11 of this section. (Note that Pa2 = 0 in the calculation of Δη in Equation 10 of this section.)
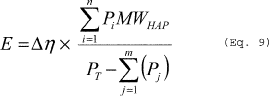 Where: E = Mass of HAP emitted. Δη = The number of moles of
noncondensable displaced from the vessel, as calculated using
Equation 10 of this section. PT = Pressure in the receiver. Pi =
Partial pressure of the individual HAP determined at the exit
temperature of the condenser or at the conditions of the dedicated
receiver. Pj = Partial pressure of the individual condensable
(including HAP) determined at the exit temperature of the condenser
or at the conditions of the dedicated receiver. n = Number of HAP
compounds in the emission stream. i = Identifier for a HAP
compound. j = Identifier for a condensable compound. MWHAP = The
average molecular weight of HAP in vapor exiting the dedicated
receiver, as calculated using Equation 11 of this section with
partial pressures determined at the exit temperature and exit
pressure conditions of the condenser or at the conditions of the
dedicated receiver. m = Number of condensable compounds (including
HAP) in the emission stream.
Where: E = Mass of HAP emitted. Δη = The number of moles of
noncondensable displaced from the vessel, as calculated using
Equation 10 of this section. PT = Pressure in the receiver. Pi =
Partial pressure of the individual HAP determined at the exit
temperature of the condenser or at the conditions of the dedicated
receiver. Pj = Partial pressure of the individual condensable
(including HAP) determined at the exit temperature of the condenser
or at the conditions of the dedicated receiver. n = Number of HAP
compounds in the emission stream. i = Identifier for a HAP
compound. j = Identifier for a condensable compound. MWHAP = The
average molecular weight of HAP in vapor exiting the dedicated
receiver, as calculated using Equation 11 of this section with
partial pressures determined at the exit temperature and exit
pressure conditions of the condenser or at the conditions of the
dedicated receiver. m = Number of condensable compounds (including
HAP) in the emission stream. 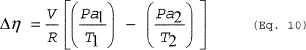
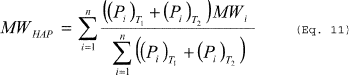 Where: Δη =
Number of moles of noncondensable gas displaced from the vessel. V
= Volume of free space in the vessel. R = Ideal gas law constant.
T1 = Initial temperature of vessel contents, absolute. T2 = Final
temperature of vessel contents, absolute. Pan = Partial pressure of
noncondensable gas in the vessel headspace at initial (n = 1) and
final (n = 2) temperature. MWHAP = The average molecular weight of
HAP in vapor exiting the dedicated receiver. (Pi)Tn = Partial
pressure of each HAP in the vessel headspace at initial (T1) and
final (T2) temperature of the receiver. MWi = Molecular weight of
the individual HAP. n = Number of HAP compounds in the emission
stream. i = Identifier for a HAP compound.
Where: Δη =
Number of moles of noncondensable gas displaced from the vessel. V
= Volume of free space in the vessel. R = Ideal gas law constant.
T1 = Initial temperature of vessel contents, absolute. T2 = Final
temperature of vessel contents, absolute. Pan = Partial pressure of
noncondensable gas in the vessel headspace at initial (n = 1) and
final (n = 2) temperature. MWHAP = The average molecular weight of
HAP in vapor exiting the dedicated receiver. (Pi)Tn = Partial
pressure of each HAP in the vessel headspace at initial (T1) and
final (T2) temperature of the receiver. MWi = Molecular weight of
the individual HAP. n = Number of HAP compounds in the emission
stream. i = Identifier for a HAP compound.
(ii) While boiling, the vessel must be operated with a properly operated process condenser. An initial demonstration that a process condenser is properly operated must be conducted during the boiling operation and documented in the notification of compliance status report described in § 63.11985(a). You must either measure the liquid temperature in the receiver or the temperature of the gas stream exiting the condenser and show it is less than the boiling or bubble point of the HAP in the vessel; or perform a material balance around the vessel and condenser and show that at least 99 percent of the recovered HAP vaporized while boiling is condensed. This demonstration is not required if the process condenser is followed by a condenser acting as a control device or if the control device is monitored using a CEMS.
(d) Depressurization. You must calculate emissions from depressurization using Equation 12 of this section.
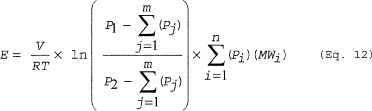 Where: E = Emissions. V = Free volume in vessel being
depressurized. R = Ideal gas law constant. T = Temperature of the
vessel, absolute. P1 = Initial pressure in the vessel. P2 = Final
pressure in the vessel. Pj = Partial pressure of the individual
condensable compounds (including HAP). MWi = Molecular weight of
the individual HAP compounds. n = Number of HAP compounds in the
emission stream. m = Number of condensable compounds (including
HAP) in the emission stream. i = Identifier for a HAP compound. j =
Identifier for a condensable compound. ln = Natural logarithm.
Where: E = Emissions. V = Free volume in vessel being
depressurized. R = Ideal gas law constant. T = Temperature of the
vessel, absolute. P1 = Initial pressure in the vessel. P2 = Final
pressure in the vessel. Pj = Partial pressure of the individual
condensable compounds (including HAP). MWi = Molecular weight of
the individual HAP compounds. n = Number of HAP compounds in the
emission stream. m = Number of condensable compounds (including
HAP) in the emission stream. i = Identifier for a HAP compound. j =
Identifier for a condensable compound. ln = Natural logarithm.
(e) Vacuum systems. You must calculate emissions from vacuum systems using Equation 13 of this section if the air leakage rate is known or can be approximated. The receiving vessel is part of the vacuum system for purposes of this subpart.
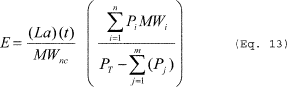 Where: E = Mass of HAP emitted. PT = Absolute pressure of
receiving vessel or ejector outlet conditions, if there is no
receiver. Pi = Partial pressure of the HAP at the receiver
temperature or the ejector outlet conditions. Pj = Partial pressure
of condensable (including HAP) at the receiver temperature or the
ejector outlet conditions. La = Total air leak rate in the system,
mass/time. MWnc = Molecular weight of noncondensable gas. t = Time
of vacuum operation. MWi = Molecular weight of the individual HAP
in the emission stream, with HAP partial pressures calculated at
the temperature of the receiver or ejector outlet, as appropriate.
(f) Gas evolution. You must calculate emissions from gas
evolution using Equation 13 in paragraph (e) of this section with
mass flow rate of gas evolution, Wg, substituted for La. (g) Air
drying. You must calculate emissions from air drying using
Equation 14 of this section:
Where: E = Mass of HAP emitted. PT = Absolute pressure of
receiving vessel or ejector outlet conditions, if there is no
receiver. Pi = Partial pressure of the HAP at the receiver
temperature or the ejector outlet conditions. Pj = Partial pressure
of condensable (including HAP) at the receiver temperature or the
ejector outlet conditions. La = Total air leak rate in the system,
mass/time. MWnc = Molecular weight of noncondensable gas. t = Time
of vacuum operation. MWi = Molecular weight of the individual HAP
in the emission stream, with HAP partial pressures calculated at
the temperature of the receiver or ejector outlet, as appropriate.
(f) Gas evolution. You must calculate emissions from gas
evolution using Equation 13 in paragraph (e) of this section with
mass flow rate of gas evolution, Wg, substituted for La. (g) Air
drying. You must calculate emissions from air drying using
Equation 14 of this section: 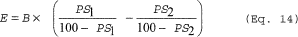 Where: E =
Mass of HAP emitted. B = Mass of dry solids. PS1 = HAP in material
entering dryer, weight percent. PS2 = HAP in material exiting
dryer, weight percent.
Where: E =
Mass of HAP emitted. B = Mass of dry solids. PS1 = HAP in material
entering dryer, weight percent. PS2 = HAP in material exiting
dryer, weight percent.
(h) Empty vessel purging. You must calculate emissions from empty vessel purging using Equation 15 of this section (Note: The term e-Ft/v can be assumed to be 0):
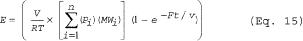 Where: V = Volume of empty vessel. R = Ideal gas law
constant. T = Temperature of the vessel vapor space; absolute. Pi =
Partial pressure of the individual HAP at the beginning of the
purge. MWi = Molecular weight of the individual HAP. F = Flow rate
of the purge gas. t = Duration of the purge. n = Number of HAP
compounds in the emission stream. i = Identifier for a HAP
compound.
Where: V = Volume of empty vessel. R = Ideal gas law
constant. T = Temperature of the vessel vapor space; absolute. Pi =
Partial pressure of the individual HAP at the beginning of the
purge. MWi = Molecular weight of the individual HAP. F = Flow rate
of the purge gas. t = Duration of the purge. n = Number of HAP
compounds in the emission stream. i = Identifier for a HAP
compound.
(i) Engineering assessments. You must conduct an engineering assessment to calculate HAP emissions or emission episodes from each process vent that are not due to vapor displacement, partially filled vessel purging, heating, depressurization, vacuum operations, gas evolution, air drying or empty vessel purging. An engineering assessment may also be used to support a finding that the emissions estimation equations in this section are inappropriate. All data, assumptions and procedures used in the engineering assessment must be documented, are subject to preapproval by the Administrator, and must be reported in the batch precompliance report. An engineering assessment should include, but is not limited to, the items listed in paragraphs (i)(1) through (4) of this section.
(1) Previous test results provided the tests are representative of current operating practices at the process unit.
(2) Bench-scale or pilot-scale test data representative of the process under representative operating conditions.
(3) Maximum flow rate, HAP emission rate, concentration, or other relevant parameter specified or implied within a permit limit applicable to the process vent.
(4) Design analysis based on accepted chemical engineering principles, measurable process parameters, or physical or chemical laws or properties. Examples of analytical methods include, but are not limited to the following:
(i) Use of material balances based on process stoichiometry to estimate maximum organic HAP concentrations.
(ii) Estimation of maximum flow rate based on physical equipment design such as pump or blower capacities.
(iii) Estimation of HAP concentrations based on saturation conditions.