Appendix A to Subpart I of Part 98 - Alternative Procedures for Measuring Point-of-Use Abatement Device Destruction or Removal Efficiency
40:23.0.1.1.3.9.1.10.39 : Appendix A
Appendix A to Subpart I of Part 98 - Alternative Procedures for
Measuring Point-of-Use Abatement Device Destruction or Removal
Efficiency
If you are measuring destruction or removal efficiency of a
point-of-use abatement device according to EPA 430-R-10-003
(incorporated by reference, see § 98.7) as specified in §
98.94(f)(4), you may follow the alternative procedures specified in
paragraphs (a) through (c) of this appendix.
(a) In place of the Quadrupole Mass Spectrometry protocol
requirements specified in section 2.2.4 of EPA 430-R-10-003
(incorporated by reference, see § 98.7), you must conduct mass
spectrometry testing in accordance with the provisions in paragraph
(a)(1) through (a)(15) of this appendix.
(1) Detection limits. The mass spectrometer chosen for
this application must have the necessary sensitivity to detect the
selected effluent species at or below the maximum field detection
limits specified in Table 3 of section 2.2.7 of EPA 430-R-10-003
(incorporated by reference, see § 98.7).
(2) Sampling location. The sample at the inlet of the
point-of-use abatement device must be taken downstream of the
process tool and pump package. The sample exhaust must be vented
back into the corrosive house ventilation system at a point
downstream of the sample inlet location.
(3) Sampling conditions. For etch processes, destruction
or removal efficiencies must be determined while etching a
substrate (product, dummy, or test). For chemical vapor deposition
processes, destruction or removal efficiencies must be determined
during a chamber clean after deposition (destruction or removal
efficiencies must not be determined in a clean chamber). All
sampling must be performed non-intrusively during wafer processing.
Samples must be drawn through the mass spectrometer source by an
external sample pump. Because of the volatility, vapor pressure,
stability and inertness of CF4, C2F6, C3F8, CHF3, NF3, and SF6, the
sample lines do not need to be heated.
(4) Mass spectrometer parameters. The specific mass spectrometer
operating conditions such as electron energy, secondary electron
multiplier voltage, emission current, and ion focusing voltage must
be selected according to the specifications provided by the mass
spectrometer manufacturer, the mass spectrometer system manual,
basic mass spectrometer textbook, or other such sources. The mass
spectrometer responses to each of the target analytes must all be
calibrated under the same mass spectrometer operating
conditions.
(5) Flow rates. A sample flow rate of 0.5-1.5 standard
liters per minute (slm) must be drawn from the process tool exhaust
stream under study.
(6) Sample frequency. The mass spectrometer sampling
frequency for etch processes must be in the range of 0.5 to 1
cycles per second, and for chemical vapor deposition processes must
be in the range of 0.25 to 0.5 cycles per second. As an alternative
you may use the sampling frequencies specified in section 2.2.4 of
EPA 430-R-10-003 (incorporated by reference, see § 98.7).
(7) Dynamic dilution calibration parameters. The
quadrupole mass spectrometer must be calibrated for both mass
location and response to analytes. A dynamic dilution calibration
system may be used to perform both types of mass spectrometer
system calibrations using two mass flow controllers. Use one mass
flow controller to regulate the flow rate of the standard component
used to calibrate the system and the second mass flow controller to
regulate the amount of diluent gas used to mix with the standard to
generate the calibration curve for each compound of interest. The
mass flow controller must be calibrated using the single component
gas being used with them, for example, nitrogen (N2) for the
diluent. A mass flow controller used with calibration mixtures must
be calibrated with the calibration mixture balance gas (for
example, N2 or He) if the analyte components are 2 percent or less
of the volume of the sample. All calibration mixtures must be
National Institute of Standards and Technology Traceable gases or
equivalent. They must be calibrated over their range of use and
must be operated in their experimentally determined dynamic linear
range. If compressed gas standards cannot be brought into the fab,
metered gas flows of target compounds into the process chamber,
under no thermal or plasma conditions and with no wafer(s) present,
and with no process emissions from other tools contributing to the
sample location, must then be performed throughout the appropriate
concentration ranges to derive calibration curves for the
subsequent destruction or removal efficiency tests.
(8) Mass location calibration. A mixture containing 1
percent He, Ar, Kr, and Xe in a balance gas of nitrogen must be
used to assure the alignment of the quadrupole mass filter (see EPA
Method 205 at 40 CFR part 51, appendix M as reference). The mass
spectrometer must be chosen so that the mass range is sufficient to
detect the predominant peaks of the components under study.
(9) Quadrupole mass spectrometer response calibration. A
calibration curve must be generated for each compound of
interest.
(10) Calibration frequency. The mass spectrometer must be
calibrated at the start of testing a given process. The calibration
must be checked at the end of testing.
(11) Calibration range. The mass spectrometer must be
calibrated over the expected concentration range of analytes using
a minimum of five concentrations including a zero. The zero point
is defined as diluent containing no added analyte.
(12) Operating procedures. You must follow the operating
procedures specified in paragraphs (a)(12)(i) through (v) of this
appendix.
(i) You must perform a qualitative mass calibration by running a
standard (or by flowing chamber gases under non-process conditions)
containing stable components such as Ar, Kr, and Xe that provide
predominant signals at m/e values distributed throughout the mass
range to be used. You must adjust the quadrupole mass filter as
needed to align with the inert gas fragments.
(ii) You must quantitatively calibrate the quadrupole mass
spectrometer for each analyte of interest. The analyte
concentrations during calibration must include the expected
concentrations in the process effluent. The calibration must be
performed under the same operating conditions, such as inlet
pressure, as when sampling process exhaust. If the calibration
inlet pressure differs from the sampling inlet pressure then the
relationship between inlet pressure and quadrupole mass
spectrometer signal response must be empirically determined and
applied to correct for any differences between calibration and
process emissions monitoring data.
(iii) To determine the response time of the instrument to
changes in a process, a process gas such as C2F6 must be turned on
at the process tool for a fixed period of time (for example, 20
seconds), after which the gas is shut off. The sample flow rate
through the system must be adjusted so that the signal increases to
a constant concentration within a few seconds and decreases to
background levels also within a few seconds.
(iv) You must sample the process effluent through the quadrupole
mass spectrometer and acquire data for the required amount of time
to track the process, as determined in paragraph (a)(12)(iii) of
this appendix. You must set the sample frequency to monitor the
changes in the process as specified in paragraph (a)(6) of this
appendix. You must repeat this for at least five substrates on the
same process and calculate the average and standard deviation of
the analyte concentration.
(v) You must repeat the quantitative calibration at the
conclusion of sampling to identify any drifts in quadrupole mass
spectrometer sensitivity. If drift is observed, you must use an
internal standard to correct for changes in sensitivity.
(13) Sample analysis. To determine the concentration of a
specific component in the sample, you must divide the ion intensity
of the sample response by the calibrated response factor for each
component.
(14) Deconvolution of interfering peaks. The effects of
interfering peaks must be deconvoluted from the mass spectra for
each target analyte.
(15) Calculations. Plot ion intensity versus analyte
concentration for a given compound obtained when calibrating the
analytical system. Determine the slope and intercept for each
calibrated species to obtain response factors with which to
calculate concentrations in the sample. For an acceptable
calibration, the R 2 value of the calibration curve must be at
least 0.98.
(b) In place of the Fourier Transform Infrared Spectroscopy
protocol requirements specified in section 2.2.4 of EPA
430-R-10-003 (incorporated by reference, see § 98.7), you may
conduct Fourier Transform Infrared Spectroscopy testing in
accordance with the provisions in paragraph (b)(1) through (17) of
this appendix, including the laboratory study phase described in
paragraphs (b)(1) through (7), and the field study phase described
in paragraphs (b)(8) through (17) of this appendix.
(1) Conformance with provisions associated with the
Calibration Transfer Standard. This procedure calls for the use
of a calibration transfer standard in a number of instances. The
use of a calibration transfer standard is necessary to validate
optical pathlength and detector response for spectrometers where
cell temperature, cell pressure, and cell optical pathlength are
potentially variable. For fixed pathlength spectrometers capable of
controlling cell temperature and pressure to within ±10 percent of
a desired set point, the use of a calibration transfer standard, as
described in paragraphs (b)(2) to (17) this appendix is not
required.
(2) Defining spectroscopic conditions. Define a set of
spectroscopic conditions under which the field studies and
subsequent field applications are to be carried out. These include
the minimum instrumental line-width, spectrometer wave number
range, sample gas temperature, sample gas pressure, absorption
pathlength, maximum sampling system volume (including the
absorption cell), minimum sample flow rate, and maximum allowable
time between consecutive infrared analyses of the effluent.
(3) Criteria for reference spectral libraries. On the
basis of previous emissions test results and/or process knowledge
(including the documentation of results of any initial and
subsequent tests, and the final reports required in §
98.97(d)(4)(i)), estimate the maximum concentrations of all of the
analytes in the effluent and their minimum concentrations of
interest (those concentrations below which the measurement of the
compounds is of no importance to the analysis). Values between the
maximum expected concentration and the minimum concentration of
interest are referred to below as the “expected concentration
range.” A minimum of three reference spectra is sufficient for a
small expected concentration range (e.g., a difference of 30
percent of the range between the low and high ends of the range),
but a minimum of four spectra are needed where the range is
greater, especially for concentration ranges that may differ by
orders of magnitude. If the measurement method is not linear then
multiple linear ranges may be necessary. If this approach is
adopted, then linear range must be demonstrated to pass the
required quality control. When the set of spectra is ordered
according to absorbance, the absorbance levels of adjacent
reference spectra should not differ by more than a factor of six.
Reference spectra for each analyte should be available at
absorbance levels that bracket the analyte's expected concentration
range; minimally, the spectrum whose absorbance exceeds each
analyte's expected maximum concentration or is within 30 percent of
it must be available. The reference spectra must be collected at or
near the same temperature and pressure at which the sample is to be
analyzed under. The gas sample pressure and temperature must be
continuously monitored during field testing and you must correct
for differences in temperature and pressure between the sample and
reference spectra. Differences between the sample and reference
spectra conditions must not exceed 50 percent for pressure and 40
°C for temperature.
(4) Spectra without reference libraries. If reference
spectral libraries meeting the criteria in paragraph (b)(3) of this
appendix do not exist for all the analytes and interferants or
cannot be accurately generated from existing libraries exhibiting
lower minimum instrumental line-width values than those proposed
for the testing, prepare the required spectra according to the
procedures specified in paragraphs (b)(4)(i) and (ii) of this
appendix.
(i) Reference spectra at the same absorbance level (to within 10
percent) of independently prepared samples must be recorded. The
reference samples must be prepared from neat forms of the analyte
or from gas standards of the highest quality commonly available
from commercial sources. Either barometric or volumetric methods
may be used to dilute the reference samples to the required
concentrations, and the equipment used must be independently
calibrated to ensure suitable accuracy. Dynamic and static
reference sample preparation methods are acceptable, but dynamic
preparations must be used for reactive analytes. Any well
characterized absorption pathlength may be employed in recording
reference spectra, but the temperature and pressure of the
reference samples should match as closely as possible those of the
proposed spectroscopic conditions.
(ii) If a mercury cadmium telluride or other potentially
non-linear detector (i.e., a detector whose response vs. total
infrared power is not a linear function over the range of responses
employed) is used for recording the reference spectra, you must
correct for the effects of this type of response on the resulting
concentration values. As needed, spectra of a calibration transfer
standard must be recorded with the laboratory spectrometer system
to verify the absorption pathlength and other aspects of the system
performance. All reference spectral data must be recorded in
interferometric form and stored digitally.
(5) Sampling system preparation. Construct a sampling
system suitable for delivering the proposed sample flow rate from
the effluent source to the infrared absorption cell. For the
compounds of interest, the surfaces of the system exposed to the
effluent stream may need to be stainless steel or Teflon; because
of the potential for generation of inorganic automated gases, glass
surfaces within the sampling system and absorption cell may need to
be Teflon-coated. The sampling system should be able to deliver a
volume of sample that results in a necessary response time.
(6) Preliminary analytical routines. For the proposed
absorption pathlength to be used in actual emissions testing, you
must prepare an analysis method containing of all the effluent
compounds at their expected maximum concentrations plus the field
calibration transfer standard compound at 20 percent of its full
concentration as needed.
(7) Documentation. The laboratory techniques used to
generate reference spectra and to convert sample spectral
information to compound concentrations must be documented. The
required level of detail for the documentation is that which allows
an independent analyst to reproduce the results from the
documentation and the stored interferometric data.
(8) Spectroscopic system performance. The performance of
the proposed spectroscopic system, sampling system, and analytical
method must be rigorously examined during and after a field study.
Several iterations of the analysis method may need to be applied
depending on observed concentrations, absorbance intensities, and
interferences. During the field study, all the sampling and
analytical procedures envisioned for future field applications must
be documented. Additional procedures not required during routine
field applications, notably dynamic spiking studies of the analyte
gases, may be performed during the field study. These additional
procedures need to be performed only once if the results are
acceptable and if the effluent sources in future field applications
prove suitably similar to those chosen for the field study. If
changes in the effluent sources in future applications are noted
and require substantial changes to the analytical equipment and/or
conditions, a separate field study must be performed for the new
set of effluent source conditions. All data recorded during the
study must be retained and documented, and all spectral information
must be permanently stored in interferometric form.
(9) System installation. The spectroscopic and sampling
sub-systems must be assembled and installed according to the
manufacturers' recommendations. For the field study, the length of
the sample lines used must not be less than the maximum length
envisioned for future field applications. The system must be given
sufficient time to stabilize before testing begins.
(10) Pre-Test calibration. Record a suitable background
spectrum using pure nitrogen gas; alternatively, if the analytes of
interest are in a sample matrix consistent with ambient air, it is
beneficial to use an ambient air background to control
interferences from water and carbon dioxide. For variable
pathlength Fourier Transform Infrared Spectrometers, introduce a
sample of the calibration transfer standard gas directly into the
absorption cell at the expected sample pressure and record its
absorbance spectrum (the “initial field calibration transfer
standard spectrum”). Compare it to the laboratory calibration
transfer standard spectra to determine the effective absorption
pathlength. If possible, record spectra of field calibration gas
standards (single component standards of the analyte compounds) and
determine their concentrations using the reference spectra and
analytical routines developed in paragraphs (b)(2) through (7) of
this appendix; these spectra may be used instead of the reference
spectra in actual concentration and uncertainty calculations.
(11) Deriving the calibration transfer standard gas from tool
chamber gases. The calibration transfer standard gas may be
derived by flowing appropriate semiconductor tool chamber gases
under non-process conditions (no thermal or plasma conditions and
with no wafer(s) present) if compressed gas standards cannot be
brought on-site.
(12) Reactivity and response time checks. While sampling
ambient air and continuously recording absorbance spectra, suddenly
replace the ambient air flow with calibration transfer standard gas
introduced as close as possible to the probe tip. Examine the
subsequent spectra to determine whether the flow rate and sample
volume allow the system to respond quickly enough to changes in the
sampled gas. Should a corrosive or reactive gas be of interest in
the sample matrix it would be beneficial to determine the
reactivity in a similar fashion, if practical. Examine the
subsequent spectra to ensure that the reactivities of the analytes
with the exposed surfaces of the sampling system do not limit the
time response of the analytical system. If a pressure correction
routine is not automated, monitor the absorption cell temperature
and pressure; verify that the (absolute) pressure remains within 2
percent of the pressure specified in the proposed system
conditions.
(13) Analyte spiking. Analyte spiking must be performed.
While sampling actual source effluent, introduce a known flow rate
of calibration transfer standard gas into the sample stream as
close as possible to the probe tip or between the probe and
extraction line. Measure and monitor the total sample flow rate,
and adjust the spike flow rate until it represents 10 percent to 20
percent of the total flow rate. After waiting until at least four
absorption cell volumes have been sampled, record four spectra of
the spiked effluent, terminate the calibration transfer standard
spike flow, pause until at least four cell volumes are sampled, and
then record four (unspiked) spectra. Repeat this process until 12
spiked and 12 unspiked spectra have been obtained. If a pressure
correction routine is not automated, monitor the absorption cell
temperature and pressure; verify that the pressure remains within 2
percent of the pressure specified in the proposed system
conditions. Calculate the expected calibration transfer standard
compound concentrations in the spectra and compare them to the
values observed in the spectrum. This procedure is best performed
using a spectroscopic tracer to calculate dilution (as opposed to
measured flow rates) of the injected calibration transfer standard
(or analyte). The spectroscopic tracer should be a component not in
the gas matrix that is easily detectable and maintains a linear
absorbance over a large concentration range. Repeat this spiking
process with all effluent compounds that are potentially reactive
with either the sampling system components or with other effluent
compounds. The gas spike is delivered by a mass flow controller,
and the expected concentration of analyte of interest
(AOITheoretical) is calculated as follows:
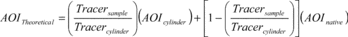
Where:
AOITheoretical = Theoretical analyte of interest concentration
(parts per million (ppm)). Tracersample = Tracer concentration
(ppm) as seen by the Fourier Transform Infrared Spectrometer during
spiking. Tracercylinder = The concentration (ppm) of tracer
recorded during direct injection of the cylinder to the Fourier
Transform Infrared Spectrometer cell. AOIcylinder = The
supplier-certified concentration (ppm) of the analyte of interest
gas standard. AOInative = The native AOI concentration (ppm) of the
effluent during stable conditions.
(14) Post-test calibration. At the end of a sampling run
and at the end of the field study, record the spectrum of the
calibration transfer standard gas. The resulting “final field
calibration transfer standard spectrum” must be compared to the
initial field calibration transfer standard spectrum to verify
suitable stability of the spectroscopic system throughout the
course of the field study.
(15) Amendment of analytical routines. The presence of
unanticipated interferant compounds and/or the observation of
compounds at concentrations outside their expected concentration
ranges may necessitate the repetition of portions of the procedures
in paragraphs (b)(2) through (14) of this appendix. Such amendments
are allowable before final analysis of the data, but must be
represented in the documentation required in paragraph (b)(16) of
this appendix.
(16) Documentation. The sampling and spiking techniques
used to generate the field study spectra and to convert sample
spectral information to concentrations must be documented at a
level of detail that allows an independent analyst to reproduce the
results from the documentation and the stored interferometric
data.
(17) Method application. When the required laboratory and
field studies have been completed and if the results indicate a
suitable degree of accuracy, the methods developed may be applied
to practical field measurement tasks. During field applications,
the procedures demonstrated in the field study specified in
paragraphs (b)(8) through (16) of this appendix must be adhered to
as closely as possible, with the following exceptions specified in
paragraphs (b)(17)(i) through (iii) of this appendix:
(i) The sampling lines employed should be as short as
practically possible and not longer than those used in the field
study.
(ii) Analyte spiking and reactivity checks are required after
the installation of or major repair to the sampling system or major
change in sample matrix. In these cases, perform three
spiked/unspiked samples with calibration transfer standard or a
surrogate analyte on a daily basis if time permits and gas
standards are easy to obtain and get on-site.
(iii) Sampling and other operational data must be recorded and
documented as during the field study, but only the interferometric
data needed to sufficiently reproduce actual test and spiking data
must be stored permanently. The format of this data does not need
to be interferograms but may be absorbance spectra or single
beams.
(c) When using the flow and dilution measurement protocol
specified in section 2.2.6 of EPA 430-R-10-003 (incorporated by
reference, see § 98.7), you may determine point-of-use abatement
device total volume flow with the modifications specified in
paragraphs (c)(1) through (3) of this appendix.
(1) You may introduce the non-reactive, non-native gas used for
determining total volume flow and dilution across the point-of-use
abatement device at a location in the exhaust of the point-of-use
abatement device. For abatement systems operating in a mode where
specific F-GHG are not readily abated, you may introduce the
non-reactive, non-native gas used for determining total volume flow
and dilution across the point-of-use abatement device prior to the
point-of-use abatement system; in this case, the tracer must be
more difficult to destroy than the target compounds being measured
based on the thermal stability of the tracer and target.
(2) You may select a location for downstream non-reactive,
non-native gas analysis that complies with the requirements in this
paragraph (c)(2) of this appendix. The sampling location should be
traversed with the sampling probe measuring the non-reactive,
non-native gas concentrations to ensure homogeneity of the
non-reactive gas and point-of-use abatement device effluent (i.e.,
stratification test). To test for stratification, measure the
non-reactive, non-native gas concentrations at three points on a
line passing through the centroidal area. Space the three points at
16.7, 50.0, and 83.3 percent of the measurement line. Sample for a
minimum of twice the system response time, determined according to
paragraph (c)(3) of this appendix, at each traverse point.
Calculate the individual point and mean non-reactive, non-native
gas concentrations. If the non-reactive, non-native gas
concentration at each traverse point differs from the mean
concentration for all traverse points by no more than ±5.0 percent
of the mean concentration, the gas stream is considered
unstratified and you may collect samples from a single point that
most closely matches the mean. If the 5.0 percent criterion is not
met, but the concentration at each traverse point differs from the
mean concentration for all traverse points by no more than ±10.0
percent of the mean, you may take samples from two points and use
the average of the two measurements. Space the two points at 16.7,
50.0, or 83.3 percent of the measurement line. If the concentration
at each traverse point differs from the mean concentration for all
traverse points by more than ±10.0 percent of the mean but less
than 20.0 percent, take samples from three points at 16.7, 50.0,
and 83.3 percent of the measurement line and use the average of the
three measurements. If the gas stream is found to be stratified
because the 20.0 percent criterion for a 3-point test is not met,
locate and sample the non-reactive, non-native gas from traverse
points for the test in accordance with Sections 11.2 and 11.3 of
EPA Method 1 in 40 CFR part 60, Appendix A-1. A minimum of 40
non-reactive gas concentration measurements will be collected at
three to five different injected non-reactive gas flow rates for
determination of point-of-use abatement device effluent flow. The
total volume flow of the point-of-use abatement device exhaust will
be calculated consistent with the EPA 430-R-10-003 (incorporated by
reference, see § 98.7) Equations 1 through 7.
(3) You must determine the measurement system response time
according to paragraphs (c)(3)(i) through (iii) of this
appendix.
(i) Before sampling begins, introduce ambient air at the probe
upstream of all sample condition components in system calibration
mode. Record the time it takes for the measured concentration of a
selected compound (for example, carbon dioxide) to reach steady
state.
(ii) Introduce nitrogen in the system calibration mode and
record the time required for the concentration of the selected
compound to reach steady state.
(iii) Observe the time required to achieve 95 percent of a
stable response for both nitrogen and ambient air. The longer
interval is the measurement system response time.
[78 FR 68234, Nov. 13, 2013]
Appendix A to Subpart L of Part 98 - Mass Balance Method for Fluorinated Gas Production
40:23.0.1.1.3.12.1.10.42 : Appendix A
Appendix A to Subpart L of Part 98 - Mass Balance Method for
Fluorinated Gas Production
1. Mass Balance Method for § 98.123(b). [Note: Numbering
convention here matches original rule text, 75 FR 74774, December
1, 2010.]
(b) Mass balance method. Before using the mass balance
approach to estimate your fluorinated GHG emissions from a process,
you must ensure that the process and the equipment and methods used
to measure it meet either the error limits described in this
paragraph and calculated under paragraph (b)(1) of this section or
the requirements specified in paragraph § 98.124(b)(8). If you
choose to calculate the error limits, you must estimate the
absolute and relative errors associated with using the mass balance
approach on that process using Equations L-1 through L-4 of this
section in conjunction with Equations L-5 through L-10 of this
section. You may use the mass-balance approach to estimate
emissions from the process if this calculation results in an
absolute error of less than or equal to 3,000 metric tons CO2e per
year or a relative error of less than or equal to 30 percent of the
estimated CO2e fluorinated GHG emissions. If you do not meet either
of the error limits or the requirements of paragraph §
98.124(b)(8), you must use the emission factor approach detailed in
paragraphs (c), (d), and (e) of this section to estimate emissions
from the process.
(1) Error calculation. To perform the calculation, you
must first calculate the absolute and relative errors associated
with the quantities calculated using either Equations L-7 through
L-10 of this section or Equation L-17 of this section.
Alternatively, you may estimate these errors based on the
variability of previous process measurements (e.g., the
variability of measurements of stream concentrations), provided
these measurements are representative of the current process and
current measurement devices and techniques. Once errors have been
calculated for the quantities in these equations, those errors must
be used to calculate the errors in Equations L-6 and L-5 of this
section. You may ignore the errors associated with Equations L-11,
L-12, and L-13 of this section.
(i) Where the measured quantity is a mass, the error in the mass
must be equated to the accuracy or precision (whichever is larger)
of the flowmeter, scale, or combination of volumetric and density
measurements at the flow rate or mass measured.
(ii) Where the measured quantity is a concentration of a stream
component, the error of the concentration must be equated to the
accuracy or precision (whichever is larger) with which you estimate
the mean concentration of that stream component, accounting for the
variability of the process, the frequency of the measurements, and
the accuracy or precision (whichever is larger) of the analytical
technique used to measure the concentration at the concentration
measured. If the variability of process measurements is used to
estimate the error, this variability shall be assumed to account
both for the variability of the process and the precision of the
analytical technique. Use standard statistical techniques such as
the student's t distribution to estimate the error of the mean of
the concentration measurements as a function of process variability
and frequency of measurement.
(iii) Equation L-1 of this section provides the general formula
for calculating the absolute errors of sums and differences where
the sum, S, is the summation of variables measured, a, b, c, etc.
(e.g., S = a + b + c):
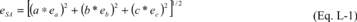
Where:
eSA = Absolute error of the sum, expressed as one half of a 95
percent confidence interval. ea = Relative error of a, expressed as
one half of a 95 percent confidence interval. eb = Relative error
of b, expressed as one half of a 95 percent confidence interval. ec
= Relative error of c, expressed as one half of a 95 percent
confidence interval.
(iv) Equation L-2 of this section provides the general formula
for calculating the relative errors of sums and differences:

Where:
eSR = Relative error of the sum, expressed as one half of a 95
percent confidence interval. eSA = Absolute error of the sum,
expressed as one half of a 95 percent confidence interval. a + b +
c = Sum of the variables measured.
(v) Equation L-3 of this section provides the general formula
for calculating the absolute errors of products (e.g., flow
rates of GHGs calculated as the product of the flow rate of the
stream and the concentration of the GHG in the stream), where the
product, P, is the result of multiplying the variables measured, a,
b, c, etc. (e.g., P = a*b*c):

Where:
ePA = Absolute error of the product, expressed as one half of a 95
percent confidence interval. ea = Relative error of a, expressed as
one half of a 95 percent confidence interval. eb = Relative error
of b, expressed as one half of a 95 percent confidence interval. ec
= Relative error of c, expressed as one half of a 95 percent
confidence interval.
(vi) Equation L-4 of this section provides the general formula
for calculating the relative errors of products:

Where:
ePR = Relative error of the product, expressed as one half of a 95
percent confidence interval. ePA = Absolute error of the product,
expressed as one half of a 95 percent confidence interval. a*b*c =
Product of the variables measured.
(vii) Calculate the absolute error of the emissions estimate in
terms of CO2e by performing a preliminary estimate of the annual
CO2e emissions of the process using the method in paragraph
(b)(1)(viii) of this section. Multiply this result by the relative
error calculated for the mass of fluorine emitted from the process
in Equation L-6 of this section.
(viii) To estimate the annual CO2e emissions of the process for
use in the error estimate, apply the methods set forth in
paragraphs (b)(2) through (7) and (b)(9) through (16) of this
section to representative process measurements. If these process
measurements represent less than one year of typical process
activity, adjust the estimated emissions to account for one year of
typical process activity. To estimate the terms FERd, FEP, and FEBk
for use in the error estimate for Equations L-11, L-12, and L-13 of
this section, you must either use emission testing, monitoring of
emitted streams, and/or engineering calculations or assessments, or
in the alternative assume that all fluorine is emitted in the form
of the fluorinated GHG that has the highest GWP among the
fluorinated GHGs that occur in more than trace concentrations in
the process. To convert the fluorinated GHG emissions to CO2e, use
Equation A-1 of § 98.2. For fluorinated GHGs whose GWPs are not
listed in Table A-1 to subpart A of this part, use a default GWP of
2,000.
(2) The total mass of each fluorinated GHG emitted annually from
each fluorinated gas production and each fluorinated GHG
transformation process must be estimated by using Equation L-5 of
this section.
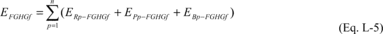
Where:
EFGHGf = Total mass of each fluorinated GHG f emitted annually from
production or transformation process i (metric tons). ERp-FGHGf =
Total mass of fluorinated GHG reactant f emitted from production
process i over the period p (metric tons, calculated in Equation
L-11 of this section). EPp-FGHGf = Total mass of the fluorinated
GHG product f emitted from production process i over the period p
(metric tons, calculated in Equation L-12 of this section).
EBp-FGHGf = Total mass of fluorinated GHG by-product f emitted from
production process i over the period p (metric tons, calculated in
Equation L-13 of this section). n = Number of concentration and
flow measurement periods for the year.
(3) The total mass of fluorine emitted from process i over the
period p must be estimated at least monthly by calculating the
difference between the total mass of fluorine in the reactant(s)
(or inputs, for processes that do not involve a chemical reaction)
and the total mass of fluorine in the product (or outputs, for
processes that do not involve a chemical reaction), accounting for
the total mass of fluorine in any destroyed or recaptured streams
that contain reactants, products, or by-products (or inputs or
outputs). This calculation must be performed using Equation L-6 of
this section. An element other than fluorine may be used in the
mass-balance equation, provided the element occurs in all of the
fluorinated GHGs fed into or generated by the process. In this
case, the mass fractions of the element in the reactants, products,
and by-products must be calculated as appropriate for that
element.
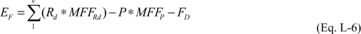
Where: EF
= Total mass of fluorine emitted from process i over the period p
(metric tons). Rd = Total mass of the fluorine-containing reactant
d that is fed into process i over the period p (metric tons). P =
Total mass of the fluorine-containing product produced by process i
over the period p (metric tons). MFFRd = Mass fraction of fluorine
in reactant d, calculated in Equation L-14 of this section. MFFP =
Mass fraction of fluorine in the product, calculated in Equation
L-15 of this section. FD = Total mass of fluorine in destroyed or
recaptured streams from process i containing fluorine-containing
reactants, products, and by-products over the period p, calculated
in Equation L-7 of this section. v = Number of fluorine-containing
reactants fed into process i.
(4) The mass of total fluorine in destroyed or recaptured
streams containing fluorine-containing reactants, products, and
by-products must be estimated at least monthly using Equation L-7
of this section unless you use the alternative approach provided in
paragraph (b)(15) of this section.
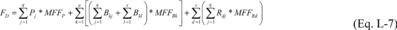
Where: FD
= Total mass of fluorine in destroyed or recaptured streams from
process i containing fluorine-containing reactants, products, and
by-products over the period p. Pj = Mass of the fluorine-containing
product removed from process i in stream j and destroyed over the
period p (calculated in Equation L-8 or L-9 of this section). Bkj =
Mass of fluorine-containing by-product k removed from process i in
stream j and destroyed over the period p (calculated in Equation
L-8 or L-9 of this section). Bkl = Mass of fluorine-containing
by-product k removed from process i in stream l and recaptured over
the period p. Rdj = Mass of fluorine-containing reactant d removed
from process i in stream j and destroyed over the period p
(calculated in Equation L-8 or L-9 of this section). MFFRd = Mass
fraction of fluorine in reactant d, calculated in Equation L-14 of
this section. MFFP = Mass fraction of fluorine in the product,
calculated in Equation L-15 of this section. MFFBk = Mass fraction
of fluorine in by-product k, calculated in Equation L-16 of this
section. q = Number of streams destroyed in process i. x = Number
of streams recaptured in process i. u = Number of
fluorine-containing by-products generated in process i. v = Number
of fluorine-containing reactants fed into process i.
(5) The mass of each fluorinated GHG removed from process i in
stream j and destroyed over the period p (i.e., Pj, Bkj, or
Rdj, as applicable) must be estimated by applying the destruction
efficiency (DE) of the device that has been demonstrated for the
fluorinated GHG f to fluorinated GHG f using Equation L-8 of this
section:

Where:
MFGHGfj = Mass of fluorinated GHG f removed from process i in
stream j and destroyed over the period p. (This may be Pj, Bkj, or
Rdj, as applicable.) DEFGHGf = Destruction efficiency of the device
that has been demonstrated for fluorinated GHG f in stream j
(fraction). CFGHGfj = Concentration (mass fraction) of fluorinated
GHG f in stream j removed from process i and fed into the
destruction device over the period p. If this concentration is only
a trace concentration, cF-GHGfj is equal to zero. Sj = Mass removed
in stream j from process i and fed into the destruction device over
the period p (metric tons).
(6) The mass of each fluorine-containing compound that is not a
fluorinated GHG and that is removed from process i in stream j and
destroyed over the period p (i.e., Pj, Bkj, or Rdj, as
applicable) must be estimated using Equation L-9 of this
section.

Where:
MFCgj = Mass of non-GHG fluorine-containing compound g removed from
process i in stream j and destroyed over the period p. (This may be
Pj, Bkj, or Rdj, as applicable). cFCgj = Concentration (mass
fraction) of non-GHG fluorine-containing compound g in stream j
removed from process i and fed into the destruction device over the
period p. If this concentration is only a trace concentration,
cFCgj is equal to zero. Sj = Mass removed in stream j from process
i and fed into the destruction device over the period p (metric
tons).
(7) The mass of fluorine-containing by-product k removed from
process i in stream l and recaptured over the period p must be
estimated using Equation L-10 of this section:

Where:
Bkl = Mass of fluorine-containing by-product k removed from process
i in stream l and recaptured over the period p (metric tons). cBkl
= Concentration (mass fraction) of fluorine-containing by-product k
in stream l removed from process i and recaptured over the period
p. If this concentration is only a trace concentration, cBkl is
equal to zero. Sl = Mass removed in stream l from process i and
recaptured over the period p (metric tons).
(8) To estimate the terms FERd, FEP, and FEBk for Equations
L-11, L-12, and L-13 of this section, you must assume that the
total mass of fluorine emitted, EF, estimated in Equation L-6 of
this section, occurs in the form of the fluorinated GHG that has
the highest GWP among the fluorinated GHGs that occur in more than
trace concentrations in the process unless you possess emission
characterization measurements showing otherwise. These emission
characterization measurements must meet the requirements in
paragraph (8)(i), (ii), or (iii) of this section, as appropriate.
The sum of the terms must equal 1. You must document the data and
calculations that are used to speciate individual compounds and to
estimate FERd, FEP, and FEBk. Exclude from your calculations the
fluorine included in FD. For example, exclude fluorine-containing
compounds that are not fluorinated GHGs and that result from the
destruction of fluorinated GHGs by any destruction devices
(e.g., the mass of HF created by combustion of an HFC).
However, include emissions of fluorinated GHGs that survive the
destruction process.
(i) If the calculations under paragraph (b)(1)(viii) of this
section, or any subsequent measurements and calculations under this
subpart, indicate that the process emits 25,000 metric tons CO2e or
more, estimate the emissions from each process vent, considering
controls, using the methods in § 98.123(c)(1). You must
characterize the emissions of any process vent that emits 25,000
metric tons CO2e or more as specified in § 98.124(b)(4).
(ii) For other vents, including vents from processes that emit
less than 25,000 metric tons CO2e, you must characterize emissions
as specified in § 98.124(b)(5).
(iii) For fluorine emissions that are not accounted for by vent
estimates, you must characterize emissions as specified in §
98.124(b)(6).
(9) The total mass of fluorine-containing reactant d emitted
must be estimated at least monthly based on the total fluorine
emitted and the fraction that consists of fluorine-containing
reactants using Equation L-11 of this section. If the
fluorine-containing reactant d is a non-GHG, you may assume that
FERd is zero.
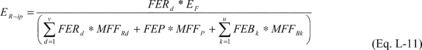
Where:
ER-ip = Total mass of fluorine-containing reactant d that is
emitted from process i over the period p (metric tons). FERd = The
fraction of the mass emitted that consists of the
fluorine-containing reactant d. EF = Total mass of fluorine
emissions from process i over the period p (metric tons),
calculated in Equation L-6 of this section. FEP = The fraction of
the mass emitted that consists of the fluorine-containing product.
FEBk = The fraction of the mass emitted that consists of
fluorine-containing by-product k. MFFRd = Mass fraction of fluorine
in reactant d, calculated in Equation L-14 of this section. MFFP =
Mass fraction of fluorine in the product, calculated in Equation
L-15 of this section. MFFBk = Mass fraction of fluorine in
by-product k, calculation in Equation L-16 of this section. u =
Number of fluorine-containing by-products generated in process i. v
= Number of fluorine-containing reactants fed into process i.
(10) The total mass of fluorine-containing product emitted must
be estimated at least monthly based on the total fluorine emitted
and the fraction that consists of fluorine-containing products
using Equation L-12 of this section. If the fluorine-containing
product is a non-GHG, you may assume that FEP is zero.
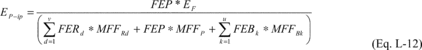
Where:
EP-ip = Total mass of fluorine-containing product emitted from
process i over the period p (metric tons). FEP = The fraction of
the mass emitted that consists of the fluorine-containing product.
EF = Total mass of fluorine emissions from process i over the
period p (metric tons), calculated in Equation L-6 of this section.
FERd = The fraction of the mass emitted that consists of
fluorine-containing reactant d. FEBk = The fraction of the mass
emitted that consists of fluorine-containing by-product k. MFFRd =
Mass fraction of fluorine in reactant d, calculated in Equation
L-14 of this section. MFFP = Mass fraction of fluorine in the
product, calculated in Equation L-15 of this section. MFFBk = Mass
fraction of fluorine in by-product k, calculation in Equation L-16
of this section. u = Number of fluorine-containing by-products
generated in process i. v = Number of fluorine-containing reactants
fed into process i.
(11) The total mass of fluorine-containing by-product k emitted
must be estimated at least monthly based on the total fluorine
emitted and the fraction that consists of fluorine-containing
by-products using Equation L-13 of this section. If
fluorine-containing by-product k is a non-GHG, you may assume that
FEBk is zero.
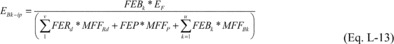
Where:
EBk-ip = Total mass of fluorine-containing by-product k emitted
from process i over the period p (metric tons). FEBk = The fraction
of the mass emitted that consists of fluorine-containing by-product
k. FERd = The fraction of the mass emitted that consists of
fluorine-containing reactant d. FEP = The fraction of the mass
emitted that consists of the fluorine-containing product. EF =
Total mass of fluorine emissions from process i over the period p
(metric tons), calculated in Equation L-6 of this section. MFFRd =
Mass fraction of fluorine in reactant d, calculated in Equation
L-14 of this section. MFFP = Mass fraction of fluorine in the
product, calculated in Equation L-15 of this section. MFFBk = Mass
fraction of fluorine in by-product k, calculation in Equation L-16
of this section. u = Number of fluorine-containing by-products
generated in process i. v = Number of fluorine-containing reactants
fed into process i.
(12) The mass fraction of fluorine in reactant d must be
estimated using Equation L-14 of this section:

Where:
MFFRd = Mass fraction of fluorine in reactant d (fraction). MFRd =
Moles fluorine per mole of reactant d. AWF = Atomic weight of
fluorine. MWRd = Molecular weight of reactant d.
(13) The mass fraction of fluorine in the product must be
estimated using Equation L-15 of this section:

Where:
MFFP = Mass fraction of fluorine in the product (fraction). MFP =
Moles fluorine per mole of product. AWF = Atomic weight of
fluorine. MWP = Molecular weight of the product produced.
(14) The mass fraction of fluorine in by-product k must be
estimated using Equation L-16 of this section:

Where:
MFFBk = Mass fraction of fluorine in the product (fraction). MFBk =
Moles fluorine per mole of by-product k. AWF = Atomic weight of
fluorine. MWBk = Molecular weight of by-product k.
(15) Alternative for determining the mass of fluorine destroyed
or recaptured. As an alternative to using Equation L-7 of this
section as provided in paragraph (b)(4) of this section, you may
estimate at least monthly the total mass of fluorine in destroyed
or recaptured streams containing fluorine-containing compounds
(including all fluorine-containing reactants, products, and
byproducts) using Equation L-17 of this section.
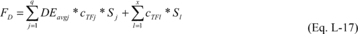
Where: FD
= Total mass of fluorine in destroyed or recaptured streams from
process i containing fluorine-containing reactants, products, and
by-products over the period p. DEavgj = Weighted average
destruction efficiency of the destruction device for the
fluorine-containing compounds identified in destroyed stream j
under § 98.124(b)(4)(ii) and (5)(ii) (calculated in Equation L-18
of this section)(fraction). cTFj = Concentration (mass fraction) of
total fluorine in stream j removed from process i and fed into the
destruction device over the period p. If this concentration is only
a trace concentration, cTFj is equal to zero. Sj = Mass removed in
stream j from process i and fed into the destruction device over
the period p (metric tons). cTFl = Concentration (mass fraction) of
total fluorine in stream l removed from process i and recaptured
over the period p. If this concentration is only a trace
concentration, cBkl is equal to zero. Sl = Mass removed in stream l
from process i and recaptured over the period p. q = Number of
streams destroyed in process i. x = Number of streams recaptured in
process i.
(16) Weighted average destruction efficiency. For purposes of
Equation L-17 of this section, calculate the weighted average
destruction efficiency applicable to a destroyed stream using
Equation L-18 of this section.
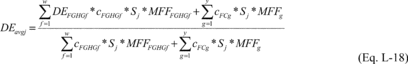
Where:
DEavgj = Weighted average destruction efficiency of the destruction
device for the fluorine-containing compounds identified in
destroyed stream j under 98.124(b)(4)(ii) or (b)(5)(ii), as
appropriate. DEFGHGf = Destruction efficiency of the device that
has been demonstrated for fluorinated GHG f in stream j (fraction).
cFGHGfj = Concentration (mass fraction) of fluorinated GHG f in
stream j removed from process i and fed into the destruction device
over the period p. If this concentration is only a trace
concentration, cF-GHGfj is equal to zero. cFCgj = Concentration
(mass fraction) of non-GHG fluorine-containing compound g in stream
j removed from process i and fed into the destruction device over
the period p. If this concentration is only a trace concentration,
cFCgj is equal to zero. Sj = Mass removed in stream j from process
i and fed into the destruction device over the period p (metric
tons). MFFFGHGf = Mass fraction of fluorine in fluorinated GHG f,
calculated in Equation L-14, L-15, or L-16 of this section, as
appropriate. MFFFCg = Mass fraction of fluorine in non-GHG
fluorine-containing compound g, calculated in Equation L-14, L-15,
or L-16 of this section, as appropriate. w = Number of fluorinated
GHGs in destroyed stream j. y = Number of non-GHG
fluorine-containing compounds in destroyed stream j.
2. Mass Balance Method for § 98.124(b). [Note: Numbering
convention here matches original rule text, 75 FR 74774, December
1, 2010.]
(b) Mass balance monitoring. If you determine fluorinated
GHG emissions from any process using the mass balance method under
§ 98.123(b), you must estimate the total mass of each fluorinated
GHG emitted from that process at least monthly. Only streams that
contain greater than trace concentrations of fluorine-containing
reactants, products, or by-products must be monitored under this
paragraph. If you use an element other than fluorine in the
mass-balance equation pursuant to § 98.123(b)(3), substitute that
element for fluorine in the monitoring requirements of this
paragraph.
(1) Mass measurements. Measure the following masses on a
monthly or more frequent basis using flowmeters, weigh scales, or a
combination of volumetric and density measurements with accuracies
and precisions that allow the facility to meet the error criteria
in § 98.123(b)(1):
(i) Total mass of each fluorine-containing product produced.
Account for any used fluorine-containing product added into the
production process upstream of the output measurement as directed
at §§ 98.413(b) and 98.414(b). For each product, the mass produced
used for the mass-balance calculation must be the same as the mass
produced that is reported under subpart OO of this part, where
applicable.
(ii) Total mass of each fluorine-containing reactant fed into
the process.
(iii) The mass removed from the process in each stream fed into
the destruction device.
(iv) The mass removed from the process in each recaptured
stream.
(2) Concentration measurements for use with §
98.123(b)(4). If you use § 98.123(b)(4) to estimate the mass of
fluorine in destroyed or recaptured streams, measure the following
concentrations at least once each calendar month during which the
process is operating, on a schedule to ensure that the measurements
are representative of the full range of process conditions
(e.g., catalyst age). Measure more frequently if this is
necessary to meet the error criteria in § 98.123(b)(1). Use
equipment and methods (e.g., gas chromatography) that comply
with paragraph (e) of this section and that have an accuracy and
precision that allow the facility to meet the error criteria in §
98.123(b)(1). Only fluorine-containing reactants, products, and
by-products that occur in a stream in greater than trace
concentrations must be monitored under this paragraph.
(i) The concentration (mass fraction) of the fluorine-containing
product in each stream that is fed into the destruction device.
(ii) The concentration (mass fraction) of each
fluorine-containing by-product in each stream that is fed into the
destruction device.
(iii) The concentration (mass fraction) of each
fluorine-containing reactant in each stream that is fed into the
destruction device.
(iv) The concentration (mass fraction) of each
fluorine-containing by-product in each stream that is recaptured
(cBkl).
(3) Concentration measurements for use with §
98.123(b)(15). If you use § 98.123(b)(15) to estimate the mass
of fluorine in destroyed or recaptured streams, measure the
concentrations listed in paragraphs (b)(3)(i) and (ii) of this
section at least once each calendar month during which the process
is operating, on a schedule to ensure that the measurements are
representative of the full range of process conditions
(e.g., catalyst age). Measure more frequently if this is
necessary to meet the error criteria in § 98.123(b)(1). Use
equipment and methods (e.g., gas chromatography) that comply
with paragraph (e) of this section and that have an accuracy and
precision that allow the facility to meet the error criteria in §
98.123(b)(1). Only fluorine-containing reactants, products, and
by-products that occur in a stream in greater than trace
concentrations must be monitored under this paragraph.
(i) The concentration (mass fraction) of total fluorine in each
stream that is fed into the destruction device.
(ii) The concentration (mass fraction) of total fluorine in each
stream that is recaptured.
(4) Emissions characterization: process vents emitting 25,000
metric tons CO2e or more. To characterize emissions from
any process vent emitting 25,000 metric tons CO2e or more, comply
with paragraphs (b)(4)(i) through (b)(4)(v) of this section, as
appropriate. Only fluorine-containing reactants, products, and
by-products that occur in a stream in greater than trace
concentrations must be monitored under this paragraph.
(i) Uncontrolled emissions. If emissions from the process
vent are not routed through a destruction device, sample and
analyze emissions at the process vent or stack or sample and
analyze emitted streams before the process vent. If the process has
more than one operating scenario, you must either perform the
emission characterization for each operating scenario or perform
the emission characterization for the operating scenario that is
expected to have the largest emissions and adjust the emission
characterization for other scenarios using engineering calculations
and assessments as specified in § 98.123(c)(4). To perform the
characterization, take three samples under conditions that are
representative for the operating scenario. Measure the
concentration of each fluorine-containing compound in each sample.
Use equipment and methods that comply with paragraph (e) of this
section. Calculate the average concentration of each
fluorine-containing compound across all three samples.
(ii) Controlled emissions using § 98.123(b)(15). If you
use § 98.123(b)(15) to estimate the total mass of fluorine in
destroyed or recaptured streams, and if the emissions from the
process vent are routed through a destruction device, characterize
emissions as specified in paragraph (b)(4)(i) of this section
before the destruction device. Apply the destruction efficiency
demonstrated for each fluorinated GHG in the destroyed stream to
that fluorinated GHG. Exclude from the characterization
fluorine-containing compounds that are not fluorinated GHGs.
(iii) Controlled emissions using § 98.123(b)(4). If you
use § 98.123(b)(4) to estimate the mass of fluorine in destroyed or
recaptured streams, and if the emissions from the process vent are
routed through a destruction device, characterize the process
vent's emissions monthly (or more frequently) using the monthly (or
more frequent) measurements under paragraphs (b)(1)(iii) and
(b)(2)(i) through (iii) of this section. Apply the destruction
efficiency demonstrated for each fluorinated GHG in the destroyed
stream to that fluorinated GHG. Exclude from the characterization
fluorine-containing compounds that are not fluorinated GHGs.
(iv) Emissions characterization frequency. You must
repeat emission characterizations performed under paragraph
(b)(4)(i) and (ii) of this section under paragraph (b)(4)(iv)(A) or
(B) of this section, whichever occurs first:
(A) 10-year revision. Repeat the emission
characterization every 10 years. In the calculations under §
98.123, apply the revised emission characterization to the process
activity that occurs after the revision.
(B) Operating scenario change that affects the emission
characterization. For planned operating scenario changes, you
must estimate and compare the emission calculation factors for the
changed operating scenario and for the original operating scenario
whose process vent specific emission factor was measured. Use the
engineering calculations and assessments specified in §
98.123(c)(4). If the share of total fluorine-containing compound
emissions represented by any fluorinated GHG changes under the
changed operating scenario by 15 percent or more of the total,
relative to the previous operating scenario (this includes the
cumulative change in the emission calculation factor since the last
emissions test), you must repeat the emission characterization.
Perform the emission characterization before February 28 of the
year that immediately follows the change. In the calculations under
§ 98.123, apply the revised emission characterization to the
process activity that occurs after the operating scenario
change.
(v) Subsequent measurements. If a process vent with
fluorinated GHG emissions less than 25,000 metric tons CO2e, per §
98.123(c)(2), is later found to have fluorinated GHG emissions of
25,000 metric tons CO2e or greater, you must perform an emission
characterization under this paragraph during the following
year.
(5) Emissions characterization: Process vents emitting less
than 25,000 metric tons CO2e. To characterize emissions
from any process vent emitting less than 25,000 metric tons CO2e,
comply with paragraphs (b)(5)(i) through (iii) of this section, as
appropriate. Only fluorine-containing reactants, products, and
by-products that occur in a stream in greater than trace
concentrations must be monitored under this paragraph.
(i) Uncontrolled emissions. If emissions from the process
vent are not routed through a destruction device, emission
measurements must consist of sampling and analysis of emissions at
the process vent or stack, sampling and analysis of emitted streams
before the process vent, previous test results, provided the tests
are representative of current operating conditions of the process,
or bench-scale or pilot-scale test data representative of the
process operating conditions.
(ii) Controlled emissions using § 98.123(b)(15). If you
use § 98.123(b)(15) to estimate the total mass of fluorine in
destroyed or recaptured streams, and if the emissions from the
process vent are routed through a destruction device, characterize
emissions as specified in paragraph (b)(5)(i) of this section
before the destruction device. Apply the destruction efficiency
demonstrated for each fluorinated GHG in the destroyed stream to
that fluorinated GHG. Exclude from the characterization
fluorine-containing compounds that are not fluorinated GHGs.
(iii) Controlled emissions using § 98.123(b)(4). If you
use § 98.123(b)(4) to estimate the mass of fluorine in destroyed or
recaptured streams, and if the emissions from the process vent are
routed through a destruction device, characterize the process
vent's emissions monthly (or more frequently) using the monthly (or
more frequent) measurements under paragraphs (b)(1)(iii) and
(b)(2)(i) through (iii) of this section. Apply the destruction
efficiency demonstrated for each fluorinated GHG in the destroyed
stream to that fluorinated GHG. Exclude from the characterization
fluorine-containing compounds that are not fluorinated GHGs.
(6) Emissions characterization: Emissions not accounted for
by process vent estimates. Calculate the weighted average
emission characterization across the process vents before any
destruction devices. Apply the weighted average emission
characterization for all the process vents to any fluorine
emissions that are not accounted for by process vent estimates.
(7) Impurities in reactants. If any fluorine-containing
impurity is fed into a process along with a reactant (or other
input) in greater than trace concentrations, this impurity shall be
monitored under this section and included in the calculations under
§ 98.123 in the same manner as reactants fed into the process, fed
into the destruction device, recaptured, or emitted, except the
concentration of the impurity in the mass fed into the process
shall be measured, and the mass of the impurity fed into the
process shall be calculated as the product of the concentration of
the impurity and the mass fed into the process. The mass of the
reactant fed into the process may be reduced to account for the
mass of the impurity.
(8) Alternative to error calculation. As an alternative
to calculating the relative and absolute errors associated with the
estimate of emissions under § 98.123(b), you may comply with the
precision, accuracy, measurement and calculation frequency, and
fluorinated GHG throughput requirements of paragraph (b)(8)(i)
through (iv) of this section.
(i) Mass measurements. Measure the masses specified in
paragraph (b)(1) of this section using flowmeters, weigh scales, or
a combination of volumetric and density measurements with
accuracies and precisions of ±0.2 percent of full scale or
better.
(ii) Concentration measurements. Measure the
concentrations specified in paragraph (b)(2) or (3) of this
section, as applicable, using analytical methods with accuracies
and precisions of ±10 percent or better.
(iii) Measurement and calculation frequency. Perform the
mass measurements specified in paragraph (b)(1) of this section and
the concentration measurements specified in paragraph (b)(2) or (3)
of this section, as applicable, at least weekly, and calculate
emissions at least weekly.
(iv) Fluorinated-GHG throughput limit. You may use the
alternative to the error calculation specified in paragraph (b)(8)
of this section only if the total annual CO2-equivalent fluorinated
GHG throughput of the process is 500,000 mtCO2e or less. The total
throughput is the sum of the masses of the fluorinated GHG
reactants, products, and by-products fed into and generated by the
process. To convert these masses to CO2e, use Equation A-1 of §
98.2. For fluorinated GHGs whose GWPs are not listed in Table A-1
to subpart A of this part, use a default GWP of 2,000.
[79 FR 73789, Dec. 11, 2014]