Appendix A to Subpart E of Part 763 - Interim Transmission Electron Microscopy Analytical Methods - Mandatory and Nonmandatory - and Mandatory Section To Determine Completion of Response Actions
40:34.0.1.1.9.2.1.17.1 : Appendix A
Appendix A to Subpart E of Part 763 - Interim Transmission Electron
Microscopy Analytical Methods - Mandatory and Nonmandatory - and
Mandatory Section To Determine Completion of Response Actions I.
Introduction
The following appendix contains three units. The first unit is
the mandatory transmission electron microscopy (TEM) method which
all laboratories must follow; it is the minimum requirement for
analysis of air samples for asbestos by TEM. The mandatory method
contains the essential elements of the TEM method. The second unit
contains the complete non-mandatory method. The non-mandatory
method supplements the mandatory method by including additional
steps to improve the analysis. EPA recommends that the
non-mandatory method be employed for analyzing air filters;
however, the laboratory may choose to employ the mandatory method.
The non-mandatory method contains the same minimum requirements as
are outlined in the mandatory method. Hence, laboratories may
choose either of the two methods for analyzing air samples by
TEM.
The final unit of this Appendix A to subpart E defines the steps
which must be taken to determine completion of response actions.
This unit is mandatory.
II. Mandatory Transmission Electron Microscopy Method A.
Definitions of Terms
1. Analytical sensitivity - Airborne asbestos
concentration represented by each fiber counted under the electron
microscope. It is determined by the air volume collected and the
proportion of the filter examined. This method requires that the
analytical sensitivity be no greater than 0.005 structures/cm
3.
2. Asbestiform - A specific type of mineral fibrosity in
which the fibers and fibrils possess high tensile strength and
flexibility.
3. Aspect ratio - A ratio of the length to the width of a
particle. Minimum aspect ratio as defined by this method is equal
to or greater than 5:1.
4. Bundle - A structure composed of three or more fibers
in a parallel arrangement with each fiber closer than one fiber
diameter.
5. Clean area - A controlled environment which is
maintained and monitored to assure a low probability of asbestos
contamination to materials in that space. Clean areas used in this
method have HEPA filtered air under positive pressure and are
capable of sustained operation with an open laboratory blank which
on subsequent analysis has an average of less than 18 structures/mm
2 in an area of 0.057 mm 2 (nominally 10 200-mesh grid openings)
and a maximum of 53 structures/mm 2 for any single preparation for
that same area.
6. Cluster - A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group. Groupings must have more than two
intersections.
7. ED - Electron diffraction.
8. EDXA - Energy dispersive X-ray analysis.
9. Fiber - A structure greater than or equal to 0.5 µm in
length with an aspect ratio (length to width) of 5:1 or greater and
having substantially parallel sides.
10. Grid - An open structure for mounting on the sample
to aid in its examination in the TEM. The term is used here to
denote a 200-mesh copper lattice approximately 3 mm in
diameter.
11. Intersection - Nonparallel touching or crossing of
fibers, with the projection having an aspect ratio of 5:1 or
greater.
12. Laboratory sample coordinator - That person
responsible for the conduct of sample handling and the
certification of the testing procedures.
13. Filter background level - The concentration of
structures per square millimeter of filter that is considered
indistinguishable from the concentration measured on a blank
(filters through which no air has been drawn). For this method the
filter background level is defined as 70 structures/mm 2.
14. Matrix - Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
15. NSD - No structure detected.
16. Operator - A person responsible for the TEM
instrumental analysis of the sample.
17. PCM - Phase contrast microscopy.
18. SAED - Selected area electron diffraction.
19. SEM - Scanning electron microscope.
20. STEM - Scanning transmission electron microscope.
21. Structure - a microscopic bundle, cluster, fiber, or
matrix which may contain asbestos.
22. S/cm 3 - Structures per cubic centimeter.
23. S/mm 2 - Structures per square millimeter.
24. TEM - Transmission electron microscope.
B. Sampling
1. The sampling agency must have written quality control
procedures and documents which verify compliance.
2. Sampling operations must be performed by qualified
individuals completely independent of the abatement contractor to
avoid possible conflict of interest (References 1, 2, 3, and 5 of
Unit II.J.).
3. Sampling for airborne asbestos following an abatement action
must use commercially available cassettes.
4. Prescreen the loaded cassette collection filters to assure
that they do not contain concentrations of asbestos which may
interfere with the analysis of the sample. A filter blank average
of less than 18 s/mm 2 in an area of 0.057 mm 2 (nominally 10
200-mesh grid openings) and a single preparation with a maximum of
53 s/mm 2 for that same area is acceptable for this method.
5. Use sample collection filters which are either polycarbonate
having a pore size less than or equal to 0.4 µm or mixed cellulose
ester having a pore size less than or equal to 0.45 µm.
6. Place these filters in series with a 5.0 µm backup filter (to
serve as a diffuser) and a support pad. See the following Figure
1:
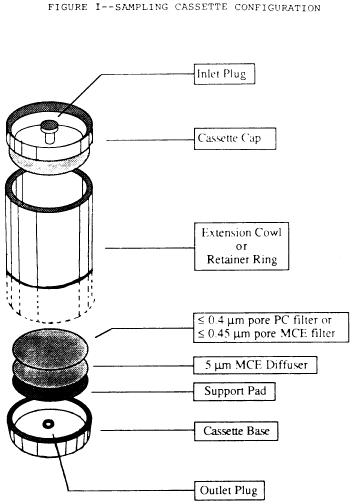
7. Reloading of used cassettes is not permitted.
8. Orient the cassette downward at approximately 45 degrees from
the horizontal.
9. Maintain a log of all pertinent sampling information.
10. Calibrate sampling pumps and their flow indicators over the
range of their intended use with a recognized standard. Assemble
the sampling system with a representative filter (not the filter
which will be used in sampling) before and after the sampling
operation.
11. Record all calibration information.
12. Ensure that the mechanical vibrations from the pump will be
minimized to prevent transferral of vibration to the cassette.
13. Ensure that a continuous smooth flow of negative pressure is
delivered by the pump by damping out any pump action fluctuations
if necessary.
14. The final plastic barrier around the abatement area remains
in place for the sampling period.
15. After the area has passed a thorough visual inspection, use
aggressive sampling conditions to dislodge any remaining dust. (See
suggested protocol in Unit III.B.7.d.)
16. Select an appropriate flow rate equal to or greater than 1
liter per minute (L/min) or less than 10 L/min for 25 mm cassettes.
Larger filters may be operated at proportionally higher flow
rates.
17. A minimum of 13 samples are to be collected for each testing
site consisting of the following:
a. A minimum of five samples per abatement area.
b. A minimum of five samples per ambient area positioned at
locations representative of the air entering the abatement
site.
c. Two field blanks are to be taken by removing the cap for not
more than 30 seconds and replacing it at the time of sampling
before sampling is initiated at the following places:
i. Near the entrance to each abatement area.
ii. At one of the ambient sites. (DO NOT leave the field blanks
open during the sampling period.)
d. A sealed blank is to be carried with each sample set. This
representative cassette is not to be opened in the field.
18. Perform a leak check of the sampling system at each indoor
and outdoor sampling site by activating the pump with the closed
sampling cassette in line. Any flow indicates a leak which must be
eliminated before initiating the sampling operation.
19. The following Table I specifies volume ranges to be
used:
20. Ensure that the sampler is turned upright before
interrupting the pump flow.
21. Check that all samples are clearly labeled and that all
pertinent information has been enclosed before transfer of the
samples to the laboratory.
22. Ensure that the samples are stored in a secure and
representative location.
23. Do not change containers if portions of these filters are
taken for other purposes.
24. A summary of Sample Data Quality Objectives is shown in the
following Table II:
C. Sample Shipment
Ship bulk samples to the analytical laboratory in a separate
container from air samples.
D. Sample Receiving
1. Designate one individual as sample coordinator at the
laboratory. While that individual will normally be available to
receive samples, the coordinator may train and supervise others in
receiving procedures for those times when he/she is not
available.
2. Bulk samples and air samples delivered to the analytical
laboratory in the same container shall be rejected.
E. Sample Preparation
1. All sample preparation and analysis shall be performed by a
laboratory independent of the abatement contractor.
2. Wet-wipe the exterior of the cassettes to minimize
contamination possibilities before taking them into the clean room
facility.
3. Perform sample preparation in a well-equipped clean
facility.
Note:
The clean area is required to have the following minimum
characteristics. The area or hood must be capable of maintaining a
positive pressure with make-up air being HEPA-filtered. The
cumulative analytical blank concentration must average less than 18
s/mm 2 in an area of 0.057 mm 2 (nominally 10 200-mesh grid
openings) and a single preparation with a maximum of 53 s/mm 2 for
that same area.
4. Preparation areas for air samples must not only be separated
from preparation areas for bulk samples, but they must be prepared
in separate rooms.
5. Direct preparation techniques are required. The object is to
produce an intact film containing the particulates of the filter
surface which is sufficiently clear for TEM analysis.
a. TEM Grid Opening Area measurement must be done as
follows:
i. The filter portion being used for sample preparation must
have the surface collapsed using an acetone vapor technique.
ii. Measure 20 grid openings on each of 20 random 200-mesh
copper grids by placing a grid on a glass and examining it under
the PCM. Use a calibrated graticule to measure the average field
diameters. From the data, calculate the field area for an average
grid opening.
iii. Measurements can also be made on the TEM at a properly
calibrated low magnification or on an optical microscope at a
magnification of approximately 400X by using an eyepiece fitted
with a scale that has been calibrated against a stage micrometer.
Optical microscopy utilizing manual or automated procedures may be
used providing instrument calibration can be verified.
b. TEM specimen preparation from polycarbonate (PC) filters.
Procedures as described in Unit III.G. or other equivalent methods
may be used.
c. TEM specimen preparation from mixed cellulose ester (MCE)
filters.
i. Filter portion being used for sample preparation must have
the surface collapsed using an acetone vapor technique or the
Burdette procedure (Ref. 7 of Unit II.J.)
ii. Plasma etching of the collapsed filter is required. The
microscope slide to which the collapsed filter pieces are attached
is placed in a plasma asher. Because plasma ashers vary greatly in
their performance, both from unit to unit and between different
positions in the asher chamber, it is difficult to specify the
conditions that should be used. Insufficient etching will result in
a failure to expose embedded filters, and too much etching may
result in loss of particulate from the surface. As an interim
measure, it is recommended that the time for ashing of a known
weight of a collapsed filter be established and that the etching
rate be calculated in terms of micrometers per second. The actual
etching time used for the particulate asher and operating
conditions will then be set such that a 1-2 µm (10 percent) layer
of collapsed surface will be removed.
iii. Procedures as described in Unit III. or other equivalent
methods may be used to prepare samples.
F. TEM Method
1. An 80-120 kV TEM capable of performing electron diffraction
with a fluorescent screen inscribed with calibrated gradations is
required. If the TEM is equipped with EDXA it must either have a
STEM attachment or be capable of producing a spot less than 250 nm
in diameter at crossover. The microscope shall be calibrated
routinely for magnification and camera constant.
2. Determination of Camera Constant and ED Pattern
Analysis. The camera length of the TEM in ED operating mode
must be calibrated before ED patterns on unknown samples are
observed. This can be achieved by using a carbon-coated grid on
which a thin film of gold has been sputtered or evaporated. A thin
film of gold is evaporated on the specimen TEM grid to obtain
zone-axis ED patterns superimposed with a ring pattern from the
polycrystalline gold film. In practice, it is desirable to optimize
the thickness of the gold film so that only one or two sharp rings
are obtained on the superimposed ED pattern. Thicker gold film
would normally give multiple gold rings, but it will tend to mask
weaker diffraction spots from the unknown fibrous particulate.
Since the unknown d-spacings of most interest in asbestos analysis
are those which lie closest to the transmitted beam, multiple gold
rings are unnecessary on zone-axis ED patterns. An average camera
constant using multiple gold rings can be determined. The camera
constant is one-half the diameter of the rings times the
interplanar spacing of the ring being measured.
3. Magnification Calibration. The magnification
calibration must be done at the fluorescent screen. The TEM must be
calibrated at the grid opening magnification (if used) and also at
the magnification used for fiber counting. This is performed with a
cross grating replica (e.g., one containing 2,160 lines/mm). Define
a field of view on the fluorescent screen either by markings or
physical boundaries. The field of view must be measurable or
previously inscribed with a scale or concentric circles (all scales
should be metric). A logbook must be maintained, and the dates of
calibration and the values obtained must be recorded. The frequency
of calibration depends on the past history of the particular
microscope. After any maintenance of the microscope that involved
adjustment of the power supplied to the lenses or the high-voltage
system or the mechanical disassembly of the electron optical column
apart from filament exchange, the magnification must be
recalibrated. Before the TEM calibration is performed, the analyst
must ensure that the cross grating replica is placed at the same
distance from the objective lens as the specimens are. For
instruments that incorporate a eucentric tilting specimen stage,
all specimens and the cross grating replica must be placed at the
eucentric position.
4. While not required on every microscope in the laboratory, the
laboratory must have either one microscope equipped with energy
dispersive X-ray analysis or access to an equivalent system on a
TEM in another laboratory.
5. Microscope settings: 80-120 kV, grid assessment 250-1,000X,
then 15,000-20,000X screen magnification for analysis.
6. Approximately one-half (0.5) of the predetermined sample area
to be analyzed shall be performed on one sample grid preparation
and the remaining half on a second sample grid preparation.
7. Individual grid openings with greater than 5 percent openings
(holes) or covered with greater than 25 percent particulate matter
or obviously having nonuniform loading must not be analyzed.
8. Reject the grid if:
a. Less than 50 percent of the grid openings covered by the
replica are intact.
b. The replica is doubled or folded.
c. The replica is too dark because of incomplete dissolution of
the filter.
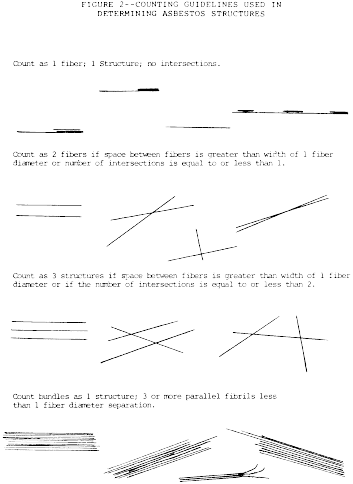
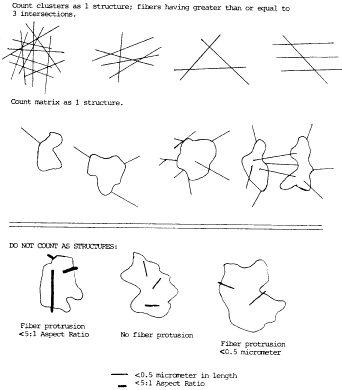
9. Recording Rules.
a. Any continuous grouping of particles in which an asbestos
fiber with an aspect ratio greater than or equal to 5:1 and a
length greater than or equal to 0.5 µm is detected shall be
recorded on the count sheet. These will be designated asbestos
structures and will be classified as fibers, bundles, clusters, or
matrices. Record as individual fibers any contiguous grouping
having 0, 1, or 2 definable intersections. Groupings having more
than 2 intersections are to be described as cluster or matrix. An
intersection is a nonparallel touching or crossing of fibers, with
the projection having an aspect ratio of 5:1 or greater. See the
following Figure 2:
i. Fiber. A structure having a minimum length greater
than or equal to 0.5 µm and an aspect ratio (length to width) of
5:1 or greater and substantially parallel sides. Note the
appearance of the end of the fiber, i.e., whether it is flat,
rounded or dovetailed.
ii. Bundle. A structure composed of three or more fibers
in a parallel arrangement with each fiber closer than one fiber
diameter.
iii. Cluster. A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group. Groupings must have more than two
intersections.
iv. Matrix. Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
b. Separate categories will be maintained for fibers less than 5
µm and for fibers equal to or greater than 5 µm in length.
c. Record NSD when no structures are detected in the field.
d. Visual identification of electron diffraction (ED) patterns
is required for each asbestos structure counted which would cause
the analysis to exceed the 70 s/mm 2 concentration. (Generally this
means the first four fibers identified as asbestos must exhibit an
identifiable diffraction pattern for chrysotile or amphibole.)
e. The micrograph number of the recorded diffraction patterns
must be reported to the client and maintained in the laboratory's
quality assurance records. In the event that examination of the
pattern by a qualified individual indicates that the pattern has
been misidentified visually, the client shall be contacted.
f. Energy Dispersive X-ray Analysis (EDXA) is required of all
amphiboles which would cause the analysis results to exceed the 70
s/mm 2 concentration. (Generally speaking, the first 4 amphiboles
would require EDXA.)
g. If the number of fibers in the nonasbestos class would cause
the analysis to exceed the 70 s/mm 2 concentration, the fact that
they are not asbestos must be confirmed by EDXA or measurement of a
zone axis diffraction pattern.
h. Fibers classified as chrysotile must be identified by
diffraction or X-ray analysis and recorded on a count sheet. X-ray
analysis alone can be used only after 70 s/mm 2 have been exceeded
for a particular sample.
i. Fibers classified as amphiboles must be identified by X-ray
analysis and electron diffraction and recorded on the count sheet.
(X-ray analysis alone can be used only after 70 s/mm 2 have been
exceeded for a particular sample.)
j. If a diffraction pattern was recorded on film, record the
micrograph number on the count sheet.
k. If an electron diffraction was attempted but no pattern was
observed, record N on the count sheet.
l. If an EDXA spectrum was attempted but not observed, record N
on the count sheet.
m. If an X-ray analysis spectrum is stored, record the file and
disk number on the count sheet.
10. Classification Rules.
a. Fiber. A structure having a minimum length greater
than or equal to 0.5 µm and an aspect ratio (length to width) of
5:1 or greater and substantially parallel sides. Note the
appearance of the end of the fiber, i.e., whether it is flat,
rounded or dovetailed.
b. Bundle. A structure composed of three or more fibers
in a parallel arrangement with each fiber closer than one fiber
diameter.
c. Cluster. A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group. Groupings must have more than two
intersections.
d. Matrix. Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
11. After finishing with a grid, remove it from the microscope,
and replace it in the appropriate grid holder. Sample grids must be
stored for a minimum of 1 year from the date of the analysis; the
sample cassette must be retained for a minimum of 30 days by the
laboratory or returned at the client's request.
G. Sample Analytical Sequence
1. Under the present sampling requirements a minimum of 13
samples is to be collected for the clearance testing of an
abatement site. These include five abatement area samples, five
ambient samples, two field blanks, and one sealed blank.
2. Carry out visual inspection of work site prior to air
monitoring.
3. Collect a minimum of 5 air samples inside the work site and 5
samples outside the work site. The indoor and outdoor samples shall
be taken during the same time period.
4. Remaining steps in the analytical sequence are contained in
Unit IV of this Appendix.
H. Reporting
1. The following information must be reported to the client for
each sample analyzed:
a. Concentration in structures per square millimeter and
structures per cubic centimeter.
b. Analytical sensitivity used for the analysis.
c. Number of asbestos structures.
d. Area analyzed.
e. Volume of air sampled (which must be initially supplied to
lab by client).
f. Copy of the count sheet must be included with the report.
g. Signature of laboratory official to indicate that the
laboratory met specifications of the method.
h. Report form must contain official laboratory identification
(e.g., letterhead).
i. Type of asbestos.
I. Quality Control/Quality Assurance Procedures (Data Quality
Indicators)
Monitoring the environment for airborne asbestos requires the
use of sensitive sampling and analysis procedures. Because the test
is sensitive, it may be influenced by a variety of factors. These
include the supplies used in the sampling operation, the
performance of the sampling, the preparation of the grid from the
filter and the actual examination of this grid in the microscope.
Each of these unit operations must produce a product of defined
quality if the analytical result is to be a reliable and meaningful
test result. Accordingly, a series of control checks and reference
standards are to be performed along with the sample analysis as
indicators that the materials used are adequate and the operations
are within acceptable limits. In this way, the quality of the data
is defined and the results are of known value. These checks and
tests also provide timely and specific warning of any problems
which might develop within the sampling and analysis operations. A
description of these quality control/quality assurance procedures
is summarized in the following Table III:
1. When the samples arrive at the laboratory, check the samples
and documentation for completeness and requirements before
initiating the analysis.
2. Check all laboratory reagents and supplies for acceptable
asbestos background levels.
3. Conduct all sample preparation in a clean room environment
monitored by laboratory blanks. Testing with blanks must also be
done after cleaning or servicing the room.
4. Prepare multiple grids of each sample.
5. Provide laboratory blanks with each sample batch. Maintain a
cumulative average of these results. If there are more than 53
fibers/mm 2 per 10 200-mesh grid openings, the system must be
checked for possible sources of contamination.
6. Perform a system check on the transmission electron
microscope daily.
7. Make periodic performance checks of magnification, electron
diffraction and energy dispersive X-ray systems as set forth in
Table III under Unit II.I.
8. Ensure qualified operator performance by evaluation of
replicate analysis and standard sample comparisons as set forth in
Table III under Unit II.I.
9. Validate all data entries.
10. Recalculate a percentage of all computations and automatic
data reduction steps as specified in Table III under Unit II.I.
11. Record an electron diffraction pattern of one asbestos
structure from every five samples that contain asbestos. Verify the
identification of the pattern by measurement or comparison of the
pattern with patterns collected from standards under the same
conditions. The records must also demonstrate that the
identification of the pattern has been verified by a qualified
individual and that the operator who made the identification is
maintaining at least an 80 percent correct visual identification
based on his measured patterns.
12. Appropriate logs or records must be maintained by the
analytical laboratory verifying that it is in compliance with the
mandatory quality assurance procedures.
J. References
For additional background information on this method, the
following references should be consulted.
1. “Guidance for Controlling Asbestos-Containing Materials in
Buildings,” EPA 560/5-85-024, June 1985.
2. “Measuring Airborne Asbestos Following an Abatement Action,”
USEPA, Office of Pollution Prevention and Toxics, EPA 600/4-85-049,
1985.
3. Small, John and E. Steel. Asbestos Standards: Materials and
Analytical Methods. N.B.S. Special Publication 619, 1982.
4. Campbell, W.J., R.L. Blake, L.L. Brown, E.E. Cather, and J.J.
Sjoberg. Selected Silicate Minerals and Their Asbestiform
Varieties. Information Circular 8751, U.S. Bureau of Mines,
1977.
5. Quality Assurance Handbook for Air Pollution Measurement
System. Ambient Air Methods, EPA 600/4-77-027a, USEPA, Office of
Research and Development, 1977.
6. Method 2A: Direct Measurement of Gas Volume through Pipes and
Small Ducts. 40 CFR Part 60 Appendix A.
7. Burdette, G.J., Health & Safety Exec. Research & Lab.
Services Div., London, “Proposed Analytical Method for
Determination of Asbestos in Air.”
8. Chatfield, E.J., Chatfield Tech. Cons., Ltd., Clark, T., PEI
Assoc., “Standard Operating Procedure for Determination of Airborne
Asbestos Fibers by Transmission Electron Microscopy Using
Polycarbonate Membrane Filters,” WERL SOP 87-1, March 5, 1987.
9. NIOSH Method 7402 for Asbestos Fibers, 12-11-86 Draft.
10. Yamate, G., Agarwall, S.C., Gibbons, R.D., IIT Research
Institute, “Methodology for the Measurement of Airborne Asbestos by
Electron Microscopy,” Draft report, USEPA Contract 68-02-3266, July
1984.
11. “Guidance to the Preparation of Quality Assurance Project
Plans,” USEPA, Office of Pollution Prevention and Toxics, 1984.
III. Nonmandatory Transmission Electron Microscopy Method A.
Definitions of Terms
1. Analytical sensitivity - Airborne asbestos
concentration represented by each fiber counted under the electron
microscope. It is determined by the air volume collected and the
proportion of the filter examined. This method requires that the
analytical sensitivity be no greater than 0.005 s/cm 3.
2. Asbestiform - A specific type of mineral fibrosity in
which the fibers and fibrils possess high tensile strength and
flexibility.
3. Aspect ratio - A ratio of the length to the width of a
particle. Minimum aspect ratio as defined by this method is equal
to or greater than 5:1.
4. Bundle - A structure composed of three or more fibers
in a parallel arrangement with each fiber closer than one fiber
diameter.
5. Clean area - A controlled environment which is
maintained and monitored to assure a low probability of asbestos
contamination to materials in that space. Clean areas used in this
method have HEPA filtered air under positive pressure and are
capable of sustained operation with an open laboratory blank which
on subsequent analysis has an average of less than 18 structures/mm
2 in an area of 0.057 mm 2 (nominally 10 200 mesh grid openings)
and a maximum of 53 structures/mm 2 for no more than one single
preparation for that same area.
6. Cluster - A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group. Groupings must have more than two
intersections.
7. ED - Electron diffraction.
8. EDXA - Energy dispersive X-ray analysis.
9. Fiber - A structure greater than or equal to 0.5 µm in
length with an aspect ratio (length to width) of 5:1 or greater and
having substantially parallel sides.
10. Grid - An open structure for mounting on the sample
to aid in its examination in the TEM. The term is used here to
denote a 200-mesh copper lattice approximately 3 mm in
diameter.
11. Intersection - Nonparallel touching or crossing of
fibers, with the projection having an aspect ratio of 5:1 or
greater.
12. Laboratory sample coordinator - That person
responsible for the conduct of sample handling and the
certification of the testing procedures.
13. Filter background level - The concentration of
structures per square millimeter of filter that is considered
indistinguishable from the concentration measured on blanks
(filters through which no air has been drawn). For this method the
filter background level is defined as 70 structures/mm 2.
14. Matrix - Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
15. NSD - No structure detected.
16. Operator - A person responsible for the TEM
instrumental analysis of the sample.
17. PCM - Phase contrast microscopy.
18. SAED - Selected area electron diffraction.
19. SEM - Scanning electron microscope.
20. STEM - Scanning transmission electron microscope.
21. Structure - a microscopic bundle, cluster, fiber, or
matrix which may contain asbestos.
22. S/cm 3 - Structures per cubic centimeter.
23. S/mm 2 - Structures per square millimeter.
24. TEM - Transmission electron microscope.
B. Sampling
1. Sampling operations must be performed by qualified
individuals completely independent of the abatement contractor to
avoid possible conflict of interest (See References 1, 2, and 5 of
Unit III.L.) Special precautions should be taken to avoid
contamination of the sample. For example, materials that have not
been prescreened for their asbestos background content should not
be used; also, sample handling procedures which do not take cross
contamination possibilities into account should not be used.
2. Material and supply checks for asbestos contamination should
be made on all critical supplies, reagents, and procedures before
their use in a monitoring study.
3. Quality control and quality assurance steps are needed to
identify problem areas and isolate the cause of the contamination
(see Reference 5 of Unit III.L.). Control checks shall be
permanently recorded to document the quality of the information
produced. The sampling firm must have written quality control
procedures and documents which verify compliance. Independent
audits by a qualified consultant or firm should be performed once a
year. All documentation of compliance should be retained
indefinitely to provide a guarantee of quality. A summary of Sample
Data Quality Objectives is shown in Table II of Unit II.B.
4. Sampling materials.
a. Sample for airborne asbestos following an abatement action
using commercially available cassettes.
b. Use either a cowling or a filter-retaining middle piece.
Conductive material may reduce the potential for particulates to
adhere to the walls of the cowl.
c. Cassettes must be verified as “clean” prior to use in the
field. If packaged filters are used for loading or preloaded
cassettes are purchased from the manufacturer or a distributor, the
manufacturer's name and lot number should be entered on all field
data sheets provided to the laboratory, and are required to be
listed on all reports from the laboratory.
d. Assemble the cassettes in a clean facility (See definition of
clean area under Unit III.A.).
e. Reloading of used cassettes is not permitted.
f. Use sample collection filters which are either polycarbonate
having a pore size of less than or equal to 0.4 µm or mixed
cellulose ester having a pore size of less than or equal to 0.45
µm.
g. Place these filters in series with a backup filter with a
pore size of 5.0 µm (to serve as a diffuser) and a support pad. See
the following Figure 1:
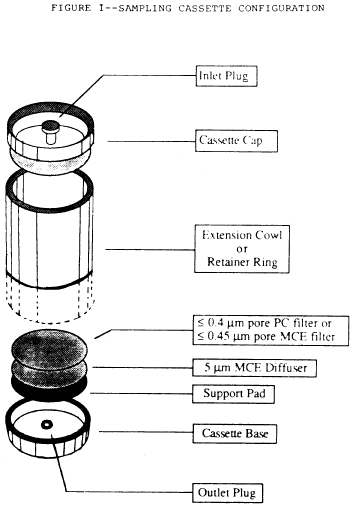
h. When polycarbonate filters are used, position the highly
reflective face such that the incoming particulate is received on
this surface.
i. Seal the cassettes to prevent leakage around the filter edges
or between cassette part joints. A mechanical press may be useful
to achieve a reproducible leak-free seal. Shrink fit gel-bands may
be used for this purpose and are available from filter
manufacturers and their authorized distributors.
j. Use wrinkle-free loaded cassettes in the sampling
operation.
5. Pump setup.
a. Calibrate the sampling pump over the range of flow rates and
loads anticipated for the monitoring period with this flow
measuring device in series. Perform this calibration using guidance
from EPA Method 2A each time the unit is sent to the field (See
Reference 6 of Unit III.L.).
b. Configure the sampling system to preclude pump vibrations
from being transmitted to the cassette by using a sampling stand
separate from the pump station and making connections with flexible
tubing.
c. Maintain continuous smooth flow conditions by damping out any
pump action fluctuations if necessary.
d. Check the sampling system for leaks with the end cap still in
place and the pump operating before initiating sample collection.
Trace and stop the source of any flow indicated by the flowmeter
under these conditions.
e. Select an appropriate flow rate equal to or greater than 1
L/min or less than 10 L/min for 25 mm cassettes. Larger filters may
be operated at proportionally higher flow rates.
f. Orient the cassette downward at approximately 45 degrees from
the horizontal.
g. Maintain a log of all pertinent sampling information, such as
pump identification number, calibration data, sample location,
date, sample identification number, flow rates at the beginning,
middle, and end, start and stop times, and other useful information
or comments. Use of a sampling log form is recommended. See the
following Figure 2:

h. Initiate a chain of custody procedure at the start of each
sampling, if this is requested by the client.
i. Maintain a close check of all aspects of the sampling
operation on a regular basis.
j. Continue sampling until at least the minimum volume is
collected, as specified in the following Table I:
k. At the conclusion of sampling, turn the cassette upward
before stopping the flow to minimize possible particle loss. If the
sampling is resumed, restart the flow before reorienting the
cassette downward. Note the condition of the filter at the
conclusion of sampling.
l. Double check to see that all information has been recorded on
the data collection forms and that the cassette is securely closed
and appropriately identified using a waterproof label. Protect
cassettes in individual clean resealed polyethylene bags. Bags are
to be used for storing cassette caps when they are removed for
sampling purposes. Caps and plugs should only be removed or
replaced using clean hands or clean disposable plastic gloves.
m. Do not change containers if portions of these filters are
taken for other purposes.
6. Minimum sample number per site. A minimum of 13 samples are
to be collected for each testing consisting of the following:
a. A minimum of five samples per abatement area.
b. A minimum of five samples per ambient area positioned at
locations representative of the air entering the abatement
site.
c. Two field blanks are to be taken by removing the cap for not
more than 30 sec and replacing it at the time of sampling before
sampling is initiated at the following places:
i. Near the entrance to each ambient area.
ii. At one of the ambient sites.
(Note:
Do not leave the blank open during the sampling period.)
d. A sealed blank is to be carried with each sample set. This
representative cassette is not to be opened in the field.
7. Abatement area sampling.
a. Conduct final clearance sampling only after the primary
containment barriers have been removed; the abatement area has been
thoroughly dried; and, it has passed visual inspection tests by
qualified personnel. (See Reference 1 of Unit III.L.)
b. Containment barriers over windows, doors, and air passageways
must remain in place until the TEM clearance sampling and analysis
is completed and results meet clearance test criteria. The final
plastic barrier remains in place for the sampling period.
c. Select sampling sites in the abatement area on a random basis
to provide unbiased and representative samples.
d. After the area has passed a thorough visual inspection, use
aggressive sampling conditions to dislodge any remaining dust.
i. Equipment used in aggressive sampling such as a leaf blower
and/or fan should be properly cleaned and decontaminated before
use.
ii. Air filtration units shall remain on during the air
monitoring period.
iii. Prior to air monitoring, floors, ceiling and walls shall be
swept with the exhaust of a minimum one (1) horsepower leaf
blower.
iv. Stationary fans are placed in locations which will not
interfere with air monitoring equipment. Fan air is directed toward
the ceiling. One fan shall be used for each 10,000 ft 3 of
worksite.
v. Monitoring of an abatement work area with high-volume pumps
and the use of circulating fans will require electrical power.
Electrical outlets in the abatement area may be used if available.
If no such outlets are available, the equipment must be supplied
with electricity by the use of extension cords and strip plug
units. All electrical power supply equipment of this type must be
approved Underwriter Laboratory equipment that has not been
modified. All wiring must be grounded. Ground fault interrupters
should be used. Extreme care must be taken to clean up any residual
water and ensure that electrical equipment does not become wet
while operational.
vi. Low volume pumps may be carefully wrapped in 6-mil
polyethylene to insulate the pump from the air. High volume pumps
cannot be sealed in this manner since the heat of the motor may
melt the plastic. The pump exhausts should be kept free.
vii. If recleaning is necessary, removal of this equipment from
the work area must be handled with care. It is not possible to
completely decontaminate the pump motor and parts since these areas
cannot be wetted. To minimize any problems in this area, all
equipment such as fans and pumps should be carefully wet wiped
prior to removal from the abatement area. Wrapping and sealing low
volume pumps in 6-mil polyethylene will provide easier
decontamination of this equipment. Use of clean water and
disposable wipes should be available for this purpose.
e. Pump flow rate equal to or greater than 1 L/min or less than
10 L/min may be used for 25 mm cassettes. The larger cassette
diameters may have comparably increased flow.
f. Sample a volume of air sufficient to ensure the minimum
quantitation limits. (See Table I of Unit III.B.5.j.)
8. Ambient sampling.
a. Position ambient samplers at locations representative of the
air entering the abatement site. If makeup air entering the
abatement site is drawn from another area of the building which is
outside of the abatement area, place the pumps in the building,
pumps should be placed out of doors located near the building and
away from any obstructions that may influence wind patterns. If
construction is in progress immediately outside the enclosure, it
may be necessary to select another ambient site. Samples should be
representative of any air entering the work site.
b. Locate the ambient samplers at least 3 ft apart and protect
them from adverse weather conditions.
c. Sample same volume of air as samples taken inside the
abatement site.
C. Sample Shipment
1. Ship bulk samples in a separate container from air samples.
Bulk samples and air samples delivered to the analytical laboratory
in the same container shall be rejected.
2. Select a rigid shipping container and pack the cassettes
upright in a noncontaminating nonfibrous medium such as a bubble
pack. The use of resealable polyethylene bags may help to prevent
jostling of individual cassettes.
3. Avoid using expanded polystyrene because of its static charge
potential. Also avoid using particle-based packaging materials
because of possible contamination.
4. Include a shipping bill and a detailed listing of samples
shipped, their descriptions and all identifying numbers or marks,
sampling data, shipper's name, and contact information. For each
sample set, designate which are the ambient samples, which are the
abatement area samples, which are the field blanks, and which is
the sealed blank if sequential analysis is to be performed.
5. Hand-carry samples to the laboratory in an upright position
if possible; otherwise choose that mode of transportation least
likely to jar the samples in transit.
6. Address the package to the laboratory sample coordinator by
name when known and alert him or her of the package description,
shipment mode, and anticipated arrival as part of the chain of
custody and sample tracking procedures. This will also help the
laboratory schedule timely analysis for the samples when they are
received.
D. Quality Control/Quality Assurance Procedures (Data Quality
Indicators)
Monitoring the environment for airborne asbestos requires the
use of sensitive sampling and analysis procedures. Because the test
is sensitive, it may be influenced by a variety of factors. These
include the supplies used in the sampling operation, the
performance of the sampling, the preparation of the grid from the
filter and the actual examination of this grid in the microscope.
Each of these unit operations must produce a product of defined
quality if the analytical result is to be a reliable and meaningful
test result. Accordingly, a series of control checks and reference
standards is performed along with the sample analysis as indicators
that the materials used are adequate and the operations are within
acceptable limits. In this way, the quality of the data is defined,
and the results are of known value. These checks and tests also
provide timely and specific warning of any problems which might
develop within the sampling and analysis operations. A description
of these quality control/quality assurance procedures is summarized
in the text below.
1. Prescreen the loaded cassette collection filters to assure
that they do not contain concentrations of asbestos which may
interfere with the analysis of the sample. A filter blank average
of less than 18 s/mm 2 in an area of 0.057 mm 2 (nominally 10
200-mesh grid openings) and a maximum of 53 s/mm 2 for that same
area for any single preparation is acceptable for this method.
2. Calibrate sampling pumps and their flow indicators over the
range of their intended use with a recognized standard. Assemble
the sampling system with a representative filter - not the filter
which will be used in sampling - before and after the sampling
operation.
3. Record all calibration information with the data to be used
on a standard sampling form.
4. Ensure that the samples are stored in a secure and
representative location.
5. Ensure that mechanical calibrations from the pump will be
minimized to prevent transferral of vibration to the cassette.
6. Ensure that a continuous smooth flow of negative pressure is
delivered by the pump by installing a damping chamber if
necessary.
7. Open a loaded cassette momentarily at one of the indoor
sampling sites when sampling is initiated. This sample will serve
as an indoor field blank.
8. Open a loaded cassette momentarily at one of the outdoor
sampling sites when sampling is initiated. This sample will serve
as an outdoor field blank.
9. Carry a sealed blank into the field with each sample series.
Do not open this cassette in the field.
10. Perform a leak check of the sampling system at each indoor
and outdoor sampling site by activating the pump with the closed
sampling cassette in line. Any flow indicates a leak which must be
eliminated before initiating the sampling operation.
11. Ensure that the sampler is turned upright before
interrupting the pump flow.
12. Check that all samples are clearly labeled and that all
pertinent information has been enclosed before transfer of the
samples to the laboratory.
E. Sample Receiving
1. Designate one individual as sample coordinator at the
laboratory. While that individual will normally be available to
receive samples, the coordinator may train and supervise others in
receiving procedures for those times when he/she is not
available.
2. Adhere to the following procedures to ensure both the
continued chain-of-custody and the accountability of all samples
passing through the laboratory:
a. Note the condition of the shipping package and data written
on it upon receipt.
b. Retain all bills of lading or shipping slips to document the
shipper and delivery time.
c. Examine the chain-of-custody seal, if any, and the package
for its integrity.
d. If there has been a break in the seal or substantive damage
to the package, the sample coordinator shall immediately notify the
shipper and a responsible laboratory manager before any action is
taken to unpack the shipment.
e. Packages with significant damage shall be accepted only by
the responsible laboratory manager after discussions with the
client.
3. Unwrap the shipment in a clean, uncluttered facility. The
sample coordinator or his or her designee will record the contents,
including a description of each item and all identifying numbers or
marks. A Sample Receiving Form to document this information is
attached for use when necessary. (See the following Figure 3.)

Note:
The person breaking the chain-of-custody seal and itemizing the
contents assumes responsibility for the shipment and signs
documents accordingly.
4. Assign a laboratory number and schedule an analysis
sequence.
5. Manage all chain-of-custody samples within the laboratory
such that their integrity can be ensured and documented.
F. Sample Preparation
1. Personnel not affiliated with the Abatement Contractor shall
be used to prepare samples and conduct TEM analysis. Wet-wipe the
exterior of the cassettes to minimize contamination possibilities
before taking them to the clean sample preparation facility.
2. Perform sample preparation in a well-equipped clean
facility.
Note:
The clean area is required to have the following minimum
characteristics. The area or hood must be capable of maintaining a
positive pressure with make-up air being HEPA filtered. The
cumulative analytical blank concentration must average less than 18
s/mm 2 in an area of 0.057 s/mm 2 (nominally 10 200-mesh grid
openings) with no more than one single preparation to exceed 53
s/mm 2 for that same area.
3. Preparation areas for air samples must be separated from
preparation areas for bulk samples. Personnel must not prepare air
samples if they have previously been preparing bulk samples without
performing appropriate personal hygiene procedures, i.e., clothing
change, showering, etc.
4. Preparation. Direct preparation techniques are
required. The objective is to produce an intact carbon film
containing the particulates from the filter surface which is
sufficiently clear for TEM analysis. Currently recommended direct
preparation procedures for polycarbonate (PC) and mixed cellulose
ester (MCE) filters are described in Unit III.F.7. and 8. Sample
preparation is a subject requiring additional research. Variation
on those steps which do not substantively change the procedure,
which improve filter clearing or which reduce contamination
problems in a laboratory are permitted.
a. Use only TEM grids that have had grid opening areas measured
according to directions in Unit III.J.
b. Remove the inlet and outlet plugs prior to opening the
cassette to minimize any pressure differential that may be
present.
c. Examples of techniques used to prepare polycarbonate filters
are described in Unit III.F.7.
d. Examples of techniques used to prepare mixed cellulose ester
filters are described in Unit III.F.8.
e. Prepare multiple grids for each sample.
f. Store the three grids to be measured in appropriately labeled
grid holders or polyethylene capsules.
5. Equipment.
a. Clean area.
b. Tweezers. Fine-point tweezers for handling of filters and TEM
grids.
c. Scalpel Holder and Curved No. 10 Surgical Blades.
d. Microscope slides.
e. Double-coated adhesive tape.
f. Gummed page reinforcements.
g. Micro-pipet with disposal tips 10 to 100 µL variable
volume.
h. Vacuum coating unit with facilities for evaporation of
carbon. Use of a liquid nitrogen cold trap above the diffusion pump
will minimize the possibility of contamination of the filter
surface by oil from the pumping system. The vacuum-coating unit can
also be used for deposition of a thin film of gold.
i. Carbon rod electrodes. Spectrochemically pure carbon
rods are required for use in the vacuum evaporator for carbon
coating of filters.
j. Carbon rod sharpener. This is used to sharpen carbon
rods to a neck. The use of necked carbon rods (or equivalent)
allows the carbon to be applied to the filters with a minimum of
heating.
k. Low-temperature plasma asher. This is used to etch the
surface of collapsed mixed cellulose ester (MCE) filters. The asher
should be supplied with oxygen, and should be modified as necessary
to provide a throttle or bleed valve to control the speed of the
vacuum to minimize disturbance of the filter. Some early models of
ashers admit air too rapidly, which may disturb particulates on the
surface of the filter during the etching step.
l. Glass petri dishes, 10 cm in diameter, 1 cm high. For
prevention of excessive evaporation of solvent when these are in
use, a good seal must be provided between the base and the lid. The
seal can be improved by grinding the base and lid together with an
abrasive grinding material.
m. Stainless steel mesh.
n. Lens tissue.
o. Copper 200-mesh TEM grids, 3 mm in diameter, or
equivalent.
p. Gold 200-mesh TEM grids, 3 mm in diameter, or equivalent.
q. Condensation washer.
r. Carbon-coated, 200-mesh TEM grids, or equivalent.
s. Analytical balance, 0.1 mg sensitivity.
t. Filter paper, 9 cm in diameter.
u. Oven or slide warmer. Must be capable of maintaining a
temperature of 65-70 °C.
v. Polyurethane foam, 6 mm thickness.
w. Gold wire for evaporation.
6. Reagents.
a. General. A supply of ultra-clean, fiber-free water
must be available for washing of all components used in the
analysis. Water that has been distilled in glass or filtered or
deionized water is satisfactory for this purpose. Reagents must be
fiber-free.
b. Polycarbonate preparation method - chloroform.
c. Mixed Cellulose Ester (MCE) preparation method - acetone or
the Burdette procedure (Ref. 7 of Unit III.L.).
7. TEM specimen preparation from polycarbonate filters.
a. Specimen preparation laboratory. It is most important
to ensure that contamination of TEM specimens by extraneous
asbestos fibers is minimized during preparation.
b. Cleaning of sample cassettes. Upon receipt at the analytical
laboratory and before they are taken into the clean facility or
laminar flow hood, the sample cassettes must be cleaned of any
contamination adhering to the outside surfaces.
c. Preparation of the carbon evaporator. If the polycarbonate
filter has already been carbon-coated prior to receipt, the carbon
coating step will be omitted, unless the analyst believes the
carbon film is too thin. If there is a need to apply more carbon,
the filter will be treated in the same way as an uncoated filter.
Carbon coating must be performed with a high-vacuum coating unit.
Units that are based on evaporation of carbon filaments in a vacuum
generated only by an oil rotary pump have not been evaluated for
this application, and must not be used. The carbon rods should be
sharpened by a carbon rod sharpener to necks of about 4 mm long and
1 mm in diameter. The rods are installed in the evaporator in such
a manner that the points are approximately 10 to 12 cm from the
surface of a microscope slide held in the rotating and tilting
device.
d. Selection of filter area for carbon coating. Before
preparation of the filters, a 75 mm × 50 mm microscope slide is
washed and dried. This slide is used to support strips of filter
during the carbon evaporation. Two parallel strips of double-sided
adhesive tape are applied along the length of the slide.
Polycarbonate filters are easily stretched during handling, and
cutting of areas for further preparation must be performed with
great care. The filter and the MCE backing filter are removed
together from the cassette and placed on a cleaned glass microscope
slide. The filter can be cut with a curved scalpel blade by rocking
the blade from the point placed in contact with the filter. The
process can be repeated to cut a strip approximately 3 mm wide
across the diameter of the filter. The strip of polycarbonate
filter is separated from the corresponding strip of backing filter
and carefully placed so that it bridges the gap between the
adhesive tape strips on the microscope slide. The filter strip can
be held with fine-point tweezers and supported underneath by the
scalpel blade during placement on the microscope slide. The analyst
can place several such strips on the same microscope slide, taking
care to rinse and wet-wipe the scalpel blade and tweezers before
handling a new sample. The filter strips should be identified by
etching the glass slide or marking the slide using a marker
insoluble in water and solvents. After the filter strip has been
cut from each filter, the residual parts of the filter must be
returned to the cassette and held in position by reassembly of the
cassette. The cassette will then be archived for a period of 30
days or returned to the client upon request.
e. Carbon coating of filter strips. The glass slide holding the
filter strips is placed on the rotation-tilting device, and the
evaporator chamber is evacuated. The evaporation must be performed
in very short bursts, separated by some seconds to allow the
electrodes to cool. If evaporation is too rapid, the strips of
polycarbonate filter will begin to curl, which will lead to
cross-linking of the surface material and make it relatively
insoluble in chloroform. An experienced analyst can judge the
thickness of carbon film to be applied, and some test should be
made first on unused filters. If the film is too thin, large
particles will be lost from the TEM specimen, and there will be few
complete and undamaged grid openings on the specimen. If the
coating is too thick, the filter will tend to curl when exposed to
chloroform vapor and the carbon film may not adhere to the support
mesh. Too thick a carbon film will also lead to a TEM image that is
lacking in contrast, and the ability to obtain ED patterns will be
compromised. The carbon film should be as thin as possible and
remain intact on most of the grid openings of the TEM specimen
intact.
f. Preparation of the Jaffe washer. The precise design of the
Jaffe washer is not considered important, so any one of the
published designs may be used. A washer consisting of a simple
stainless steel bridge is recommended. Several pieces of lens
tissue approximately 1.0 cm × 0.5 cm are placed on the stainless
steel bridge, and the washer is filled with chloroform to a level
where the meniscus contacts the underside of the mesh, which
results in saturation of the lens tissue. See References 8 and 10
of Unit III.L.
g. Placing of specimens into the Jaffe washer. The TEM grids are
first placed on a piece of lens tissue so that individual grids can
be picked up with tweezers. Using a curved scalpel blade, the
analyst excises three 3 mm square pieces of the carbon-coated
polycarbonate filter from the filter strip. The three squares are
selected from the center of the strip and from two points between
the outer periphery of the active surface and the center. The piece
of filter is placed on a TEM specimen grid with the shiny side of
the TEM grid facing upwards, and the whole assembly is placed
boldly onto the saturated lens tissue in the Jaffe washer. If
carbon-coated grids are used, the filter should be placed
carbon-coated side down. The three excised squares of filters are
placed on the same piece of lens tissue. Any number of separate
pieces of lens tissue may be placed in the same Jaffe washer. The
lid is then placed on the Jaffe washer, and the system is allowed
to stand for several hours, preferably overnight.
h. Condensation washing. It has been found that many
polycarbonate filters will not dissolve completely in the Jaffe
washer, even after being exposed to chloroform for as long as 3
days. This problem becomes more serious if the surface of the
filter was overheated during the carbon evaporation. The presence
of undissolved filter medium on the TEM preparation leads to
partial or complete obscuration of areas of the sample, and fibers
that may be present in these areas of the specimen will be
overlooked; this will lead to a low result. Undissolved filter
medium also compromises the ability to obtain ED patterns. Before
they are counted, TEM grids must be examined critically to
determine whether they are adequately cleared of residual filter
medium. It has been found that condensation washing of the grids
after the initial Jaffe washer treatment, with chloroform as the
solvent, clears all residual filter medium in a period of
approximately 1 hour. In practice, the piece of lens tissue
supporting the specimen grids is transferred to the cold finger of
the condensation washer, and the washer is operated for about 1
hour. If the specimens are cleared satisfactorily by the Jaffe
washer alone, the condensation washer step may be unnecessary.
8. TEM specimen preparation from MCE filters.
a. This method of preparing TEM specimens from MCE filters is
similar to that specified in NIOSH Method 7402. See References 7,
8, and 9 of Unit III.L.
b. Upon receipt at the analytical laboratory, the sample
cassettes must be cleaned of any contamination adhering to the
outside surfaces before entering the clean sample preparation
area.
c. Remove a section from any quadrant of the sample and blank
filters.
d. Place the section on a clean microscope slide. Affix the
filter section to the slide with a gummed paged reinforcement or
other suitable means. Label the slide with a water and
solvent-proof marking pen.
e. Place the slide in a petri dish which contains several paper
filters soaked with 2 to 3 mL acetone. Cover the dish. Wait 2 to 4
minutes for the sample filter to fuse and clear.
f. Plasma etching of the collapsed filter is required.
i. The microscope slide to which the collapsed filter pieces are
attached is placed in a plasma asher. Because plasma ashers vary
greatly in their performance, both from unit to unit and between
different positions in the asher chamber, it is difficult to
specify the conditions that should be used. This is one area of the
method that requires further evaluation. Insufficient etching will
result in a failure to expose embedded filters, and too much
etching may result in loss of particulate from the surface. As an
interim measure, it is recommended that the time for ashing of a
known weight of a collapsed filter be established and that the
etching rate be calculated in terms of micrometers per second. The
actual etching time used for a particular asher and operating
conditions will then be set such that a 1-2 µm (10 percent) layer
of collapsed surface will be removed.
ii. Place the slide containing the collapsed filters into a
low-temperature plasma asher, and etch the filter.
g. Transfer the slide to a rotating stage inside the bell jar of
a vacuum evaporator. Evaporate a 1 mm × 5 mm section of graphite
rod onto the cleared filter. Remove the slide to a clean, dry,
covered petri dish.
h. Prepare a second petri dish as a Jaffe washer with the
wicking substrate prepared from filter or lens paper placed on top
of a 6 mm thick disk of clean spongy polyurethane foam. Cut a
V-notch on the edge of the foam and filter paper. Use the V-notch
as a reservoir for adding solvent. The wicking substrate should be
thin enough to fit into the petri dish without touching the
lid.
i. Place carbon-coated TEM grids face up on the filter or lens
paper. Label the grids by marking with a pencil on the filter paper
or by putting registration marks on the petri dish lid and marking
with a waterproof marker on the dish lid. In a fume hood, fill the
dish with acetone until the wicking substrate is saturated. The
level of acetone should be just high enough to saturate the filter
paper without creating puddles.
j. Remove about a quarter section of the carbon-coated filter
samples from the glass slides using a surgical knife and tweezers.
Carefully place the section of the filter, carbon side down, on the
appropriately labeled grid in the acetone-saturated petri dish.
When all filter sections have been transferred, slowly add more
solvent to the wedge-shaped trough to bring the acetone level up to
the highest possible level without disturbing the sample
preparations. Cover the petri dish. Elevate one side of the petri
dish by placing a slide under it. This allows drops of condensed
solvent vapors to form near the edge rather than in the center
where they would drip onto the grid preparation.
G. TEM Method
1. Instrumentation.
a. Use an 80-120 kV TEM capable of performing electron
diffraction with a fluorescent screen inscribed with calibrated
gradations. If the TEM is equipped with EDXA it must either have a
STEM attachment or be capable of producing a spot less than 250 nm
in diameter at crossover. The microscope shall be calibrated
routinely (see Unit III.J.) for magnification and camera
constant.
b. While not required on every microscope in the laboratory, the
laboratory must have either one microscope equipped with energy
dispersive X-ray analysis or access to an equivalent system on a
TEM in another laboratory. This must be an Energy Dispersive X-ray
Detector mounted on TEM column and associated hardware/software to
collect, save, and read out spectral information. Calibration of
Multi-Channel Analyzer shall be checked regularly for A1 at 1.48
KeV and Cu at 8.04 KeV, as well as the manufacturer's
procedures.
i. Standard replica grating may be used to determine
magnification (e.g., 2160 lines/mm).
ii. Gold standard may be used to determine camera constant.
c. Use a specimen holder with single tilt and/or double tilt
capabilities.
2. Procedure.
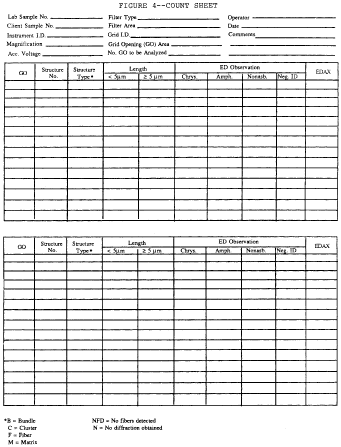
a. Start a new Count Sheet for each sample to be analyzed.
Record on count sheet: analyst's initials and date; lab sample
number; client sample number microscope identification;
magnification for analysis; number of predetermined grid openings
to be analyzed; and grid identification. See the following Figure
4:
b. Check that the microscope is properly aligned and calibrated
according to the manufacturer's specifications and
instructions.
c. Microscope settings: 80-120 kV, grid assessment 250-1000X,
then 15,000-20,000X screen magnification for analysis.
d. Approximately one-half (0.5) of the predetermined sample area
to be analyzed shall be performed on one sample grid preparation
and the remaining half on a second sample grid preparation.
e. Determine the suitability of the grid.
i. Individual grid openings with greater than 5 percent openings
(holes) or covered with greater than 25 percent particulate matter
or obviously having nonuniform loading shall not be analyzed.
ii. Examine the grid at low magnification (<1000X) to
determine its suitability for detailed study at higher
magnifications.
iii. Reject the grid if:
(1) Less than 50 percent of the grid openings covered by the
replica are intact.
(2) It is doubled or folded.
(3) It is too dark because of incomplete dissolution of the
filter.
iv. If the grid is rejected, load the next sample grid.
v. If the grid is acceptable, continue on to Step 6 if mapping
is to be used; otherwise proceed to Step 7.
f. Grid Map (Optional).
i. Set the TEM to the low magnification mode.
ii. Use flat edge or finder grids for mapping.
iii. Index the grid openings (fields) to be counted by marking
the acceptable fields for one-half (0.5) of the area needed for
analysis on each of the two grids to be analyzed. These may be
marked just before examining each grid opening (field), if
desired.
iv. Draw in any details which will allow the grid to be properly
oriented if it is reloaded into the microscope and a particular
field is to be reliably identified.
g. Scan the grid.
i. Select a field to start the examination.
ii. Choose the appropriate magnification (15,000 to 20,000X
screen magnification).
iii. Scan the grid as follows.
(1) At the selected magnification, make a series of parallel
traverses across the field. On reaching the end of one traverse,
move the image one window and reverse the traverse.
Note:
A slight overlap should be used so as not to miss any part of
the grid opening (field).
(2) Make parallel traverses until the entire grid opening
(field) has been scanned.
h. Identify each structure for appearance and size.
i. Appearance and size: Any continuous grouping of particles in
which an asbestos fiber within aspect ratio greater than or equal
to 5:1 and a length greater than or equal to 0.5 µm is detected
shall be recorded on the count sheet. These will be designated
asbestos structures and will be classified as fibers, bundles,
clusters, or matrices. Record as individual fibers any contiguous
grouping having 0, 1, or 2 definable intersections. Groupings
having more than 2 intersections are to be described as cluster or
matrix. See the following Figure 5:
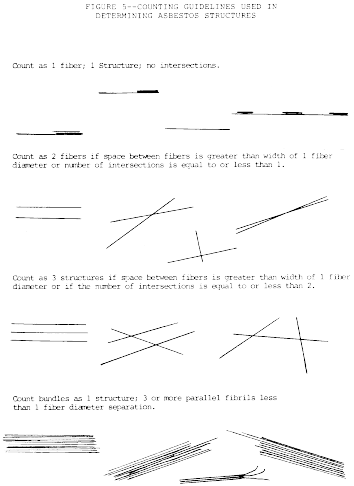
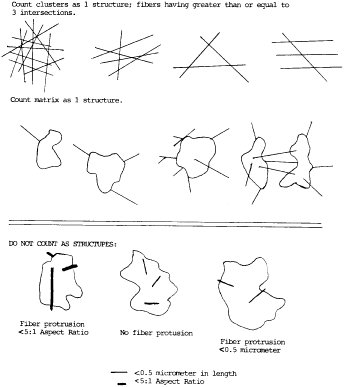
An intersection is
a non-parallel touching or crossing of fibers, with the projection
having an aspect ratio of 5:1 or greater. Combinations such as a
matrix and cluster, matrix and bundle, or bundle and cluster are
categorized by the dominant fiber quality - cluster, bundle, and
matrix, respectively. Separate categories will be maintained for
fibers less than 5 µm and for fibers greater than or equal to 5 µm
in length. Not required, but useful, may be to record the fiber
length in 1 µm intervals. (Identify each structure morphologically
and analyze it as it enters the “window”.)
(1) Fiber. A structure having a minimum length greater
than 0.5 µm and an aspect ratio (length to width) of 5:1 or greater
and substantially parallel sides. Note the appearance of the end of
the fiber, i.e., whether it is flat, rounded or dovetailed, no
intersections.
(2) Bundle. A structure composed of 3 or more fibers in a
parallel arrangement with each fiber closer than one fiber
diameter.
(3) Cluster. A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group; groupings must have more than 2
intersections.
(4) Matrix. Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
(5) NSD. Record NSD when no structures are detected in
the field.
(6) Intersection. Non-parallel touching or crossing of
fibers, with the projection having an aspect ratio 5:1 or
greater.
ii. Structure Measurement.
(1) Recognize the structure that is to be sized.
(2) Memorize its location in the “window” relative to the sides,
inscribed square and to other particulates in the field so this
exact location can be found again when scanning is resumed.
(3) Measure the structure using the scale on the screen.
(4) Record the length category and structure type classification
on the count sheet after the field number and fiber number.
(5) Return the fiber to its original location in the window and
scan the rest of the field for other fibers; if the direction of
travel is not remembered, return to the right side of the field and
begin the traverse again.
i. Visual identification of Electron Diffraction (ED) patterns
is required for each asbestos structure counted which would cause
the analysis to exceed the 70 s/mm 2 concentration. (Generally this
means the first four fibers identified as asbestos must exhibit an
identifiable diffraction pattern for chrysotile or amphibole.)
i. Center the structure, focus, and obtain an ED pattern. (See
Microscope Instruction Manual for more detailed instructions.)
ii. From a visual examination of the ED pattern, obtained with a
short camera length, classify the observed structure as belonging
to one of the following classifications: chrysotile, amphibole, or
nonasbestos.
(1) Chrysotile: The chrysotile asbestos pattern has
characteristic streaks on the layer lines other than the central
line and some streaking also on the central line. There will be
spots of normal sharpness on the central layer line and on
alternate lines (2nd, 4th, etc.). The repeat distance between layer
lines is 0.53 nm and the center doublet is at 0.73 nm. The pattern
should display (002), (110), (130) diffraction maxima; distances
and geometry should match a chrysotile pattern and be measured
semiquantitatively.
(2) Amphibole Group [includes grunerite (amosite), crocidolite,
anthophyllite, tremolite, and actinolite]: Amphibole asbestos fiber
patterns show layer lines formed by very closely spaced dots, and
the repeat distance between layer lines is also about 0.53 nm.
Streaking in layer lines is occasionally present due to crystal
structure defects.
(3) Nonasbestos: Incomplete or unobtainable ED patterns, a
nonasbestos EDXA, or a nonasbestos morphology.
iii. The micrograph number of the recorded diffraction patterns
must be reported to the client and maintained in the laboratory's
quality assurance records. The records must also demonstrate that
the identification of the pattern has been verified by a qualified
individual and that the operator who made the identification is
maintaining at least an 80 percent correct visual identification
based on his measured patterns. In the event that examination of
the pattern by the qualified individual indicates that the pattern
had been misidentified visually, the client shall be contacted. If
the pattern is a suspected chrysotile, take a photograph of the
diffraction pattern at 0 degrees tilt. If the structure is
suspected to be amphibole, the sample may have to be tilted to
obtain a simple geometric array of spots.
j. Energy Dispersive X-Ray Analysis (EDXA).
i. Required of all amphiboles which would cause the analysis
results to exceed the 70 s/mm 2 concentration. (Generally speaking,
the first 4 amphiboles would require EDXA.)
ii. Can be used alone to confirm chrysotile after the 70 s/mm 2
concentration has been exceeded.
iii. Can be used alone to confirm all nonasbestos.
iv. Compare spectrum profiles with profiles obtained from
asbestos standards. The closest match identifies and categorizes
the structure.
v. If the EDXA is used for confirmation, record the properly
labeled spectrum on a computer disk, or if a hard copy, file with
analysis data.
vi. If the number of fibers in the nonasbestos class would cause
the analysis to exceed the 70 s/mm 2 concentration, their
identities must be confirmed by EDXA or measurement of a zone axis
diffraction pattern to establish that the particles are
nonasbestos.
k. Stopping Rules.
i. If more than 50 asbestiform structures are counted in a
particular grid opening, the analysis may be terminated.
ii. After having counted 50 asbestiform structures in a minimum
of 4 grid openings, the analysis may be terminated. The grid
opening in which the 50th fiber was counted must be completed.
iii. For blank samples, the analysis is always continued until
10 grid openings have been analyzed.
iv. In all other samples the analysis shall be continued until
an analytical sensitivity of 0.005 s/cm 3 is reached.
l. Recording Rules. The count sheet should contain the following
information:
i. Field (grid opening): List field number.
ii. Record “NSD” if no structures are detected.
iii. Structure information.
(1) If fibers, bundles, clusters, and/or matrices are found,
list them in consecutive numerical order, starting over with each
field.
(2) Length. Record length category of asbestos fibers examined.
Indicate if less than 5 µm or greater than or equal to 5 µm.
(3) Structure Type. Positive identification of asbestos fibers
is required by the method. At least one diffraction pattern of each
fiber type from every five samples must be recorded and compared
with a standard diffraction pattern. For each asbestos fiber
reported, both a morphological descriptor and an identification
descriptor shall be specified on the count sheet.
(4) Fibers classified as chrysotile must be identified by
diffraction and/or X-ray analysis and recorded on the count sheet.
X-ray analysis alone can be used as sole identification only after
70s/mm 2 have been exceeded for a particular sample.
(5) Fibers classified as amphiboles must be identified by X-ray
analysis and electron diffraction and recorded on the count sheet.
(X-ray analysis alone can be used as sole identification only after
70s/mm 2 have been exceeded for a particular sample.)
(6) If a diffraction pattern was recorded on film, the
micrograph number must be indicated on the count sheet.
(7) If an electron diffraction was attempted and an appropriate
spectra is not observed, N should be recorded on the count
sheet.
(8) If an X-ray analysis is attempted but not observed, N should
be recorded on the count sheet.
(9) If an X-ray analysis spectrum is stored, the file and disk
number must be recorded on the count sheet.
m. Classification Rules.
i. Fiber. A structure having a minimum length greater
than or equal to 0.5 µm and an aspect ratio (length to width) of
5:1 or greater and substantially parallel sides. Note the
appearance of the end of the fiber, i.e., whether it is flat,
rounded or dovetailed.
ii. Bundle. A structure composed of three or more fibers
in a parallel arrangement with each fiber closer than one fiber
diameter.
iii. Cluster. A structure with fibers in a random
arrangement such that all fibers are intermixed and no single fiber
is isolated from the group. Groupings must have more than two
intersections.
iv. Matrix. Fiber or fibers with one end free and the
other end embedded in or hidden by a particulate. The exposed fiber
must meet the fiber definition.
v. NSD. Record NSD when no structures are detected in the
field.
n. After all necessary analyses of a particle structure have
been completed, return the goniometer stage to 0 degrees, and
return the structure to its original location by recall of the
original location.
o. Continue scanning until all the structures are identified,
classified and sized in the field.
p. Select additional fields (grid openings) at low
magnification; scan at a chosen magnification (15,000 to 20,000X
screen magnification); and analyze until the stopping rule becomes
applicable.
q. Carefully record all data as they are being collected, and
check for accuracy.
r. After finishing with a grid, remove it from the microscope,
and replace it in the appropriate grid hold. Sample grids must be
stored for a minimum of 1 year from the date of the analysis; the
sample cassette must be retained for a minimum of 30 days by the
laboratory or returned at the client's request.
H. Sample Analytical Sequence
1. Carry out visual inspection of work site prior to air
monitoring.
2. Collect a minimum of five air samples inside the work site
and five samples outside the work site. The indoor and outdoor
samples shall be taken during the same time period.
3. Analyze the abatement area samples according to this
protocol. The analysis must meet the 0.005 s/cm 3 analytical
sensitivity.
4. Remaining steps in the analytical sequence are contained in
Unit IV. of this Appendix.
I. Reporting
The following information must be reported to the client. See
the following Table II:
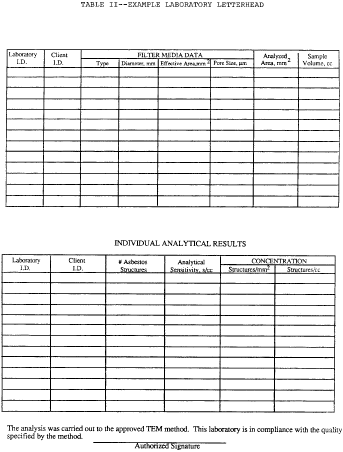
1. Concentration in structures per square millimeter and
structures per cubic centimeter.
2. Analytical sensitivity used for the analysis.
3. Number of asbestos structures.
4. Area analyzed.
5. Volume of air samples (which was initially provided by
client).
6. Average grid size opening.
7. Number of grids analyzed.
8. Copy of the count sheet must be included with the report.
9. Signature of laboratory official to indicate that the
laboratory met specifications of the AHERA method.
10. Report form must contain official laboratory identification
(e.g., letterhead).
11. Type of asbestos.
J. Calibration Methodology Note:
Appropriate implementation of the method requires a person
knowledgeable in electron diffraction and mineral identification by
ED and EDXA. Those inexperienced laboratories wishing to develop
capabilities may acquire necessary knowledge through analysis of
appropriate standards and by following detailed methods as
described in References 8 and 10 of Unit III.L.
1. Equipment Calibration. In this method, calibration is
required for the air-sampling equipment and the transmission
electron microscope (TEM).
a. TEM Magnification. The magnification at the
fluorescent screen of the TEM must be calibrated at the grid
opening magnification (if used) and also at the magnification used
for fiber counting. This is performed with a cross grating replica.
A logbook must be maintained, and the dates of calibration depend
on the past history of the particular microscope; no frequency is
specified. After any maintenance of the microscope that involved
adjustment of the power supplied to the lenses or the high-voltage
system or the mechanical disassembly of the electron optical column
apart from filament exchange, the magnification must be
recalibrated. Before the TEM calibration is performed, the analyst
must ensure that the cross grating replica is placed at the same
distance from the objective lens as the specimens are. For
instruments that incorporate an eucentric tilting specimen stage,
all speciments and the cross grating replica must be placed at the
eucentric position.
b. Determination of the TEM magnification on the fluorescent
screen.
i. Define a field of view on the fluorescent screen either by
markings or physical boundaries. The field of view must be
measurable or previously inscribed with a scale or concentric
circles (all scales should be metric).
ii. Insert a diffraction grating replica (for example a grating
containing 2,160 lines/mm) into the specimen holder and place into
the microscope. Orient the replica so that the grating lines fall
perpendicular to the scale on the TEM fluorescent screen. Ensure
that the goniometer stage tilt is 0 degrees.
iii. Adjust microscope magnification to 10,000X or 20,000X.
Measure the distance (mm) between two widely separated lines on the
grating replica. Note the number of spaces between the lines. Take
care to measure between the same relative positions on the lines
(e.g., between left edges of lines).
Note:
The more spaces included in the measurement, the more accurate
the final calculation. On most microscopes, however, the
magnification is substantially constant only within the central
8-10 cm diameter region of the fluorescent screen.
iv. Calculate the true magnification (M) on the fluorescent
screen:
M = XG/Y where: X = total distance (mm) between the designated
grating lines; G = calibration constant of the grating replica
(lines/mm): Y = number of grating replica spaces counted along X.
c. Calibration of the EDXA System. Initially, the EDXA system
must be calibrated by using two reference elements to calibrate the
energy scale of the instrument. When this has been completed in
accordance with the manufacturer's instructions, calibration in
terms of the different types of asbestos can proceed. The EDXA
detectors vary in both solid angle of detection and in window
thickness. Therefore, at a particular accelerating voltage in use
on the TEM, the count rate obtained from specific dimensions of
fiber will vary both in absolute X-ray count rate and in the
relative X-ray peak heights for different elements. Only a few
minerals are relevant for asbestos abatement work, and in this
procedure the calibration is specified in terms of a “fingerprint”
technique. The EDXA spectra must be recorded from individual fibers
of the relevant minerals, and identifications are made on the basis
of semiquantitative comparisons with these reference spectra.
d. Calibration of Grid Openings.
i. Measure 20 grid openings on each of 20 random 200-mesh copper
grids by placing a grid on a glass slide and examining it under the
PCM. Use a calibrated graticule to measure the average field
diameter and use this number to calculate the field area for an
average grid opening. Grids are to be randomly selected from
batches up to 1,000.
Note:
A grid opening is considered as one field.
ii. The mean grid opening area must be measured for the type of
specimen grids in use. This can be accomplished on the TEM at a
properly calibrated low magnification or on an optical microscope
at a magnification of approximately 400X by using an eyepiece
fitted with a scale that has been calibrated against a stage
micrometer. Optical microscopy utilizing manual or automated
procedures may be used providing instrument calibration can be
verified.
e. Determination of Camera Constant and ED Pattern Analysis.
i. The camera length of the TEM in ED operating mode must be
calibrated before ED patterns on unknown samples are observed. This
can be achieved by using a carbon-coated grid on which a thin film
of gold has been sputtered or evaporated. A thin film of gold is
evaporated on the specimen TEM grid to obtain zone-axis ED patterns
superimposed with a ring pattern from the polycrystalline gold
film.
ii. In practice, it is desirable to optimize the thickness of
the gold film so that only one or two sharp rings are obtained on
the superimposed ED pattern. Thicker gold film would normally give
multiple gold rings, but it will tend to mask weaker diffraction
spots from the unknown fibrous particulates. Since the unknown
d-spacings of most interest in asbestos analysis are those which
lie closest to the transmitted beam, multiple gold rings are
unnecessary on zone-axis ED patterns. An average camera constant
using multiple gold rings can be determined. The camera constant is
one-half the diameter, D, of the rings times the interplanar
spacing, d, of the ring being measured.
K. Quality Control/Quality Assurance Procedures (Data Quality
Indicators)
Monitoring the environment for airborne asbestos requires the
use of sensitive sampling and analysis procedures. Because the test
is sensitive, it may be influenced by a variety of factors. These
include the supplies used in the sampling operation, the
performance of the sampling, the preparation of the grid from the
filter and the actual examination of this grid in the microscope.
Each of these unit operations must produce a product of defined
quality if the analytical result is to be a reliable and meaningful
test result. Accordingly, a series of control checks and reference
standards is performed along with the sample analysis as indicators
that the materials used are adequate and the operations are within
acceptable limits. In this way, the quality of the data is defined
and the results are of known value. These checks and tests also
provide timely and specific warning of any problems which might
develop within the sampling and analysis operations. A description
of these quality control/quality assurance procedures is summarized
in the following Table III:
1. When the samples arrive at the laboratory, check the samples
and documentation for completeness and requirements before
initiating the analysis.
2. Check all laboratory reagents and supplies for acceptable
asbestos background levels.
3. Conduct all sample preparation in a clean room environment
monitored by laboratory blanks and special testing after cleaning
or servicing the room.
4. Prepare multiple grids of each sample.
5. Provide laboratory blanks with each sample batch. Maintain a
cumulative average of these results. If this average is greater
than 53 f/mm 2 per 10 200-mesh grid openings, check the system for
possible sources of contamination.
6. Check for recovery of asbestos from cellulose ester filters
submitted to plasma asher.
7. Check for asbestos carryover in the plasma asher by including
a blank alongside the positive control sample.
8. Perform a systems check on the transmission electron
microscope daily.
9. Make periodic performance checks of magnification, electron
diffraction and energy dispersive X-ray systems as set forth in
Table III of Unit III.K.
10. Ensure qualified operator performance by evaluation of
replicate counting, duplicate analysis, and standard sample
comparisons as set forth in Table III of Unit III.K.
11. Validate all data entries.
12. Recalculate a percentage of all computations and automatic
data reduction steps as specified in Table III.
13. Record an electron diffraction pattern of one asbestos
structure from every five samples that contain asbestos. Verify the
identification of the pattern by measurement or comparison of the
pattern with patterns collected from standards under the same
conditions.
The outline of quality control procedures presented above is viewed
as the minimum required to assure that quality data is produced for
clearance testing of an asbestos abated area. Additional
information may be gained by other control tests. Specifics on
those control procedures and options available for environmental
testing can be obtained by consulting References 6, 7, and 11 of
Unit III.L. L. References
For additional background information on this method the
following references should be consulted.
1. “Guidelines for Controlling Asbestos-Containing Materials in
Buildings,” EPA 560/5-85-024, June 1985.
2. “Measuring Airborne Asbestos Following an Abatement Action,”
USEP/Office of Pollution Prevention and Toxics, EPA 600/4-85-049,
1985.
3. Small, John and E. Steel. Asbestos Standards: Materials and
Analytical Methods. N.B.S. Special Publication 619, 1982.
4. Campbell, W.J., R.L. Blake, L.L. Brown, E.E. Cather, and J.J.
Sjoberg. Selected Silicate Minerals and Their Asbestiform
Varieties. Information Circular 8751, U.S. Bureau of Mines,
1977.
5. Quality Assurance Handbook for Air Pollution Measurement
System. Ambient Air Methods, EPA 600/4-77-027a, USEPA, Office of
Research and Development, 1977.
6. Method 2A: Direct Measurement of Gas Volume Through Pipes and
Small Ducts. 40 CFR Part 60 Appendix A.
7. Burdette, G.J. Health & Safety Exec., Research & Lab.
Services Div., London, “Proposed Analytical Method for
Determination of Asbestos in Air.”
8. Chatfield, E.J., Chatfield Tech. Cons., Ltd., Clark, T., PEI
Assoc. “Standard Operating Procedure for Determination of Airborne
Asbestos Fibers by Transmission Electron Microscopy Using
Polycarbonate Membrane Filters.” WERL SOP 87-1, March 5, 1987.
9. NIOSH. Method 7402 for Asbestos Fibers, December 11, 1986
Draft.
10. Yamate, G., S.C. Agarwall, R.D. Gibbons, IIT Research
Institute, “Methodology for the Measurement of Airborne Asbestos by
Electron Microscopy.” Draft report, USEPA Contract 68-02-3266, July
1984.
11. Guidance to the Preparation of Quality Assurance Project
Plans. USEPA, Office of Pollution Prevention and Toxics, 1984.
IV. Mandatory Interpretation of Transmission Electron Microscopy
Results To Determine Completion of Response Actions A. Introduction
A response action is determined to be completed by TEM when the
abatement area has been cleaned and the airborne asbestos
concentration inside the abatement area is no higher than
concentrations at locations outside the abatement area. “Outside”
means outside the abatement area, but not necessarily outside the
building. EPA reasons that an asbestos removal contractor cannot be
expected to clean an abatement area to an airborne asbestos
concentration that is lower than the concentration of air entering
the abatement area from outdoors or from other parts of the
building. After the abatement area has passed a thorough visual
inspection, and before the outer containment barrier is removed, a
minimum of five air samples inside the abatement area and a minimum
of five air samples outside the abatement area must be collected.
Hence, the response action is determined to be completed when the
average airborne asbestos concentration measured inside the
abatement area is not statistically different from the average
airborne asbestos concentration measured outside the abatement
area.
The inside and outside concentrations are compared by the
Z-test, a statistical test that takes into account the variability
in the measurement process. A minimum of five samples inside the
abatement area and five samples outside the abatement area are
required to control the false negative error rate, i.e., the
probability of declaring the removal complete when, in fact, the
air concentration inside the abatement area is significantly higher
than outside the abatement area. Additional quality control is
provided by requiring three blanks (filters through which no air
has been drawn) to be analyzed to check for unusually high filter
contamination that would distort the test results.
When volumes greater than or equal to 1,199 L for a 25 mm filter
and 2,799 L for a 37 mm filter have been collected and the average
number of asbestos structures on samples inside the abatement area
is no greater than 70 s/mm 2 of filter, the response action may be
considered complete without comparing the inside samples to the
outside samples. EPA is permitting this initial screening test to
save analysis costs in situations where the airborne asbestos
concentration is sufficiently low so that it cannot be
distinguished from the filter contamination/background level
(fibers deposited on the filter that are unrelated to the air being
sampled). The screening test cannot be used when volumes of less
than 1,199 L for 25 mm filter or 2,799 L for a 37 mm filter are
collected because the ability to distinguish levels significantly
different from filter background is reduced at low volumes.
The initial screening test is expressed in structures per square
millimeter of filter because filter background levels come from
sources other than the air being sampled and cannot be meaningfully
expressed as a concentration per cubic centimeter of air. The value
of 70 s/mm 2 is based on the experience of the panel of
microscopists who consider one structure in 10 grid openings (each
grid opening with an area of 0.0057 mm 2) to be comparable with
contamination/background levels of blank filters. The decision is
based, in part, on Poisson statistics which indicate that four
structures must be counted on a filter before the fiber count is
statistically distinguishable from the count for one structure. As
more information on the performance of the method is collected,
this criterion may be modified. Since different combinations of the
number and size of grid openings are permitted under the TEM
protocol, the criterion is expressed in structures per square
millimeter of filter to be consistent across all combinations. Four
structures per 10 grid openings corresponds to approximately 70
s/mm 2.
B. Sample Collection and Analysis
1. A minimum of 13 samples is required: five samples collected
inside the abatement area, five samples collected outside the
abatement area, two field blanks, and one sealed blank.
2. Sampling and TEM analysis must be done according to either
the mandatory or nonmandatory protocols in Appendix A. At least
0.057 mm 2 of filter must be examined on blank filters.
C. Interpretation of Results
1. The response action shall be considered complete if
either:
a. Each sample collected inside the abatement area consists of
at least 1,199 L of air for a 25 mm filter, or 2,799 L of air for a
37 mm filter, and the arithmetic mean of their asbestos structure
concentrations per square millimeter of filter is less than or
equal to 70 s/mm 2; or
b. The three blank samples have an arithmetic mean of the
asbestos structure concentration on the blank filters that is less
than or equal to 70 s/mm 2 and the average airborne asbestos
concentration measured inside the abatement area is not
statistically higher than the average airborne asbestos
concentration measured outside the abatement area as determined by
the Z-test. The Z-test is carried out by calculating
where YI is the average of the natural
logarithms of the inside samples and YO is the average of the
natural logarithms of the outside samples, nI is the number of
inside samples and nO is the number of outside samples. The
response action is considered complete if Z is less than or equal
to 1.65. Note:
When no fibers are counted, the calculated detection limit for
that analysis is inserted for the concentration.
2. If the abatement site does not satisfy either (1) or (2) of
this Section C, the site must be recleaned and a new set of samples
collected.
D. Sequence for Analyzing Samples
It is possible to determine completion of the response action
without analyzing all samples. Also, at any point in the process, a
decision may be made to terminate the analysis of existing samples,
reclean the abatement site, and collect a new set of samples. The
following sequence is outlined to minimize the number of analyses
needed to reach a decision.
1. Analyze the inside samples.
2. If at least 1,199 L of air for a 25 mm filter or 2,799 L of
air for a 37 mm filter is collected for each inside sample and the
arithmetic mean concentration of structures per square millimeter
of filter is less than or equal to 70 s/mm 2, the response action
is complete and no further analysis is needed.
3. If less than 1,199 L of air for a 25 mm filter or 2,799 L of
air for a 37 mm filter is collected for any of the inside samples,
or the arithmetic mean concentration of structures per square
millimeter of filter is greater than 70 s/mm 2, analyze the three
blanks.
4. If the arithmetic mean concentration of structures per square
millimeter on the blank filters is greater than 70 s/mm 2,
terminate the analysis, identify and correct the source of blank
contamination, and collect a new set of samples.
5. If the arithmetic mean concentration of structures per square
millimeter on the blank filters is less than or equal to 70 s/mm 2,
analyze the outside samples and perform the Z-test.
6. If the Z-statistic is less than or equal to 1.65, the
response action is complete. If the Z-statistic is greater than
1.65, reclean the abatement site and collect a new set of
samples.
[52 FR 41857, Oct. 30, 1987]