Appendix D to Part 50 - Reference Measurement Principle and Calibration Procedure for the Measurement of Ozone in the Atmosphere (Chemiluminescence Method)
40:2.0.1.1.1.0.1.20.5 : Appendix D
Appendix D to Part 50 - Reference Measurement Principle and
Calibration Procedure for the Measurement of Ozone in the
Atmosphere (Chemiluminescence Method)
1.0 Applicability.
1.1 This chemiluminescence method provides reference
measurements of the concentration of ozone (O3) in ambient air for
determining compliance with the national primary and secondary
ambient air quality standards for O3 as specified in 40 CFR part
50. This automated method is applicable to the measurement of
ambient O3 concentrations using continuous (real-time) sampling and
analysis. Additional quality assurance procedures and guidance are
provided in 40 CFR part 58, appendix A, and in Reference 14.
2.0 Measurement Principle.
2.1 This reference method is based on continuous automated
measurement of the intensity of the characteristic
chemiluminescence released by the gas phase reaction of O3 in
sampled air with either ethylene (C2H4) or nitric oxide (NO) gas.
An ambient air sample stream and a specific flowing concentration
of either C2H4 (ET-CL method) or NO (NO-CL method) are mixed in a
measurement cell, where the resulting chemiluminescence is
quantitatively measured by a sensitive photo-detector. References
8-11 describe the chemiluminescence measurement principle.
2.2 The measurement system is calibrated by referencing the
instrumental chemiluminescence measurements to certified O3
standard concentrations generated in a dynamic flow system and
assayed by photometry to be traceable to a National Institute of
Standards and Technology (NIST) standard reference photometer for
O3 (see Section 4, Calibration Procedure, below).
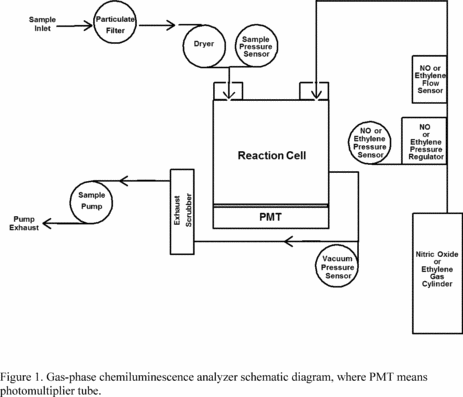
2.3 An analyzer implementing this measurement principle is shown
schematically in Figure 1. Designs implementing this measurement
principle must include: an appropriately designed mixing and
measurement cell; a suitable quantitative photometric measurement
system with adequate sensitivity and wavelength specificity for O3;
a pump, flow control, and sample conditioning system for sampling
the ambient air and moving it into and through the measurement
cell; a sample air dryer as necessary to meet the water vapor
interference limit requirement specified in subpart B of part 53 of
this chapter; a means to supply, meter, and mix a constant, flowing
stream of either C2H4 or NO gas of fixed concentration with the
sample air flow in the measurement cell; suitable electronic
control and measurement processing capability; and other associated
apparatus as may be necessary. The analyzer must be designed and
constructed to provide accurate, repeatable, and continuous
measurements of O3 concentrations in ambient air, with measurement
performance that meets the requirements specified in subpart B of
part 53 of this chapter.
2.4 An analyzer implementing this measurement principle and
calibration procedure will be considered a federal reference method
(FRM) only if it has been designated as a reference method in
accordance with part 53 of this chapter.
2.5 Sampling considerations. The use of a particle filter
on the sample inlet line of a chemiluminescence O3 FRM analyzer is
required to prevent buildup of particulate matter in the
measurement cell and inlet components. This filter must be changed
weekly (or at least often as specified in the manufacturer's
operation/instruction manual), and the sample inlet system used
with the analyzer must be kept clean, to avoid loss of O3 in the O3
sample air prior to the concentration measurement.
3.0 Interferences.
3.1 Except as described in 3.2 below, the chemiluminescence
measurement system is inherently free of significant interferences
from other pollutant substances that may be present in ambient
air.
3.2 A small sensitivity to variations in the humidity of the
sample air is minimized by a sample air dryer. Potential loss of O3
in the inlet air filter and in the air sample handling components
of the analyzer and associated exterior air sampling components due
to buildup of airborne particulate matter is minimized by filter
replacement and cleaning of the other inlet components.
4.0 Calibration Procedure.
4.1 Principle. The calibration procedure is based on the
photometric assay of O3 concentrations in a dynamic flow system.
The concentration of O3 in an absorption cell is determined from a
measurement of the amount of 254 nm light absorbed by the sample.
This determination requires knowledge of (1) the absorption
coefficient (α) of O3 at 254 nm, (2) the optical path length (l)
through the sample, (3) the transmittance of the sample at a
nominal wavelength of 254 nm, and (4) the temperature (T) and
pressure (P) of the sample. The transmittance is defined as the
ratio I/I0, where I is the intensity of light which passes through
the cell and is sensed by the detector when the cell contains an O3
sample, and I0 is the intensity of light which passes through the
cell and is sensed by the detector when the cell contains zero air.
It is assumed that all conditions of the system, except for the
contents of the absorption cell, are identical during measurement
of I and I0. The quantities defined above are related by the
Beer-Lambert absorption law,

Where: α
= absorption coefficient of O3 at 254 nm = 308 ±4 atm−1 cm−1 at 0
°C and 760 torr, 1 2 3 4 5 6 7 c = O3 concentration in atmospheres,
and l = optical path length in cm.
A stable O3 generator is used to produce O3 concentrations over
the required calibration concentration range. Each O3 concentration
is determined from the measurement of the transmittance (I/I0) of
the sample at 254 nm with a photometer of path length l and
calculated from the equation,

The calculated O3 concentrations must be corrected for O3
losses, which may occur in the photometer, and for the temperature
and pressure of the sample.
4.2 Applicability. This procedure is applicable to the
calibration of ambient air O3 analyzers, either directly or by
means of a transfer standard certified by this procedure. Transfer
standards must meet the requirements and specifications set forth
in Reference 12.
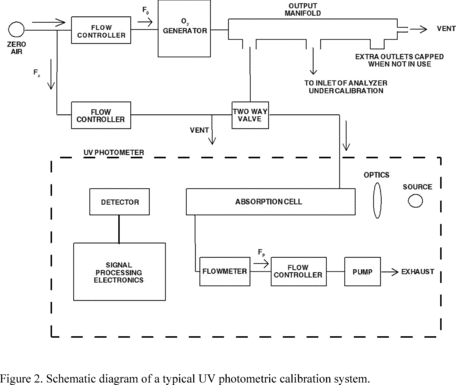
4.3 Apparatus. A complete UV calibration system consists
of an O3 generator, an output port or manifold, a photometer, an
appropriate source of zero air, and other components as necessary.
The configuration must provide a stable O3 concentration at the
system output and allow the photometer to accurately assay the
output concentration to the precision specified for the photometer
(4.3.1). Figure 2 shows a commonly used configuration and serves to
illustrate the calibration procedure, which follows. Other
configurations may require appropriate variations in the procedural
steps. All connections between components in the calibration system
downstream of the O3 generator must be of glass, Teflon, or other
relatively inert materials. Additional information regarding the
assembly of a UV photometric calibration apparatus is given in
Reference 13. For certification of transfer standards which provide
their own source of O3, the transfer standard may replace the O3
generator and possibly other components shown in Figure 2; see
Reference 12 for guidance.
4.3.1 UV photometer. The photometer consists of a
low-pressure mercury discharge lamp, (optional) collimation optics,
an absorption cell, a detector, and signal-processing electronics,
as illustrated in Figure 2. It must be capable of measuring the
transmittance, I/I0, at a wavelength of 254 nm with sufficient
precision such that the standard deviation of the concentration
measurements does not exceed the greater of 0.005 ppm or 3% of the
concentration. Because the low-pressure mercury lamp radiates at
several wavelengths, the photometer must incorporate suitable means
to assure that no O3 is generated in the cell by the lamp, and that
at least 99.5% of the radiation sensed by the detector is 254 nm
radiation. (This can be readily achieved by prudent selection of
optical filter and detector response characteristics.) The length
of the light path through the absorption cell must be known with an
accuracy of at least 99.5%. In addition, the cell and associated
plumbing must be designed to minimize loss of O3 from contact with
cell walls and gas handling components. See Reference 13 for
additional information.
4.3.2 Air flow controllers. Air flow controllers are
devices capable of regulating air flows as necessary to meet the
output stability and photometer precision requirements.
4.3.3 Ozone generator. The ozone generator used must be
capable of generating stable levels of O3 over the required
concentration range.
4.3.4 Output manifold. The output manifold must be
constructed of glass, Teflon, or other relatively inert material,
and should be of sufficient diameter to insure a negligible
pressure drop at the photometer connection and other output ports.
The system must have a vent designed to insure atmospheric pressure
in the manifold and to prevent ambient air from entering the
manifold.
4.3.5 Two-way valve. A manual or automatic two-way valve,
or other means is used to switch the photometer flow between zero
air and the O3 concentration.
4.3.6 Temperature indicator. A device to indicate
temperature must be used that is accurate to ±1 °C.
4.3.7 Barometer or pressure indicator. A device to
indicate barometric pressure must be used that is accurate to ±2
torr.
4.4 Reagents.
4.4.1 Zero air. The zero air must be free of contaminants
which would cause a detectable response from the O3 analyzer, and
it must be free of NO, C2H4, and other species which react with O3.
A procedure for generating suitable zero air is given in Reference
13. As shown in Figure 2, the zero air supplied to the photometer
cell for the I0 reference measurement must be derived from the same
source as the zero air used for generation of the O3 concentration
to be assayed (I measurement). When using the photometer to certify
a transfer standard having its own source of O3, see Reference 12
for guidance on meeting this requirement.
4.5 Procedure.
4.5.1 General operation. The calibration photometer must
be dedicated exclusively to use as a calibration standard. It must
always be used with clean, filtered calibration gases, and never
used for ambient air sampling. A number of advantages are realized
by locating the calibration photometer in a clean laboratory where
it can be stationary, protected from the physical shock of
transportation, operated by a responsible analyst, and used as a
common standard for all field calibrations via transfer
standards.
4.5.2 Preparation. Proper operation of the photometer is
of critical importance to the accuracy of this procedure. Upon
initial operation of the photometer, the following steps must be
carried out with all quantitative results or indications recorded
in a chronological record, either in tabular form or plotted on a
graphical chart. As the performance and stability record of the
photometer is established, the frequency of these steps may be
reduced to be consistent with the documented stability of the
photometer and the guidance provided in Reference 12.
4.5.2.1 Instruction manual. Carry out all set up and
adjustment procedures or checks as described in the operation or
instruction manual associated with the photometer.
4.5.2.2 System check. Check the photometer system for
integrity, leaks, cleanliness, proper flow rates, etc. Service or
replace filters and zero air scrubbers or other consumable
materials, as necessary.
4.5.2.3 Linearity. Verify that the photometer
manufacturer has adequately established that the linearity error of
the photometer is less than 3%, or test the linearity by dilution
as follows: Generate and assay an O3 concentration near the upper
range limit of the system or appropriate calibration scale for the
instrument, then accurately dilute that concentration with zero air
and re-assay it. Repeat at several different dilution ratios.
Compare the assay of the original concentration with the assay of
the diluted concentration divided by the dilution ratio, as
follows

Where: E
= linearity error, percent A1 = assay of the original concentration
A2 = assay of the diluted concentration R = dilution ratio = flow
of original concentration divided by the total flow
The linearity error must be less than 5%. Since the accuracy of
the measured flow-rates will affect the linearity error as measured
this way, the test is not necessarily conclusive. Additional
information on verifying linearity is contained in Reference
13.
4.5.2.4 Inter-comparison. The photometer must be
inter-compared annually, either directly or via transfer standards,
with a NIST standard reference photometer (SRP) or calibration
photometers used by other agencies or laboratories.
4.5.2.5 Ozone losses. Some portion of the O3 may be lost
upon contact with the photometer cell walls and gas handling
components. The magnitude of this loss must be determined and used
to correct the calculated O3 concentration. This loss must not
exceed 5%. Some guidelines for quantitatively determining this loss
are discussed in Reference 13.
4.5.3 Assay of O3 concentrations. The operator
must carry out the following steps to properly assay O3
concentrations.
4.5.3.1 Allow the photometer system to warm up and
stabilize.
4.5.3.2 Verify that the flow rate through the photometer
absorption cell, F, allows the cell to be flushed in a reasonably
short period of time (2 liter/min is a typical flow). The precision
of the measurements is inversely related to the time required for
flushing, since the photometer drift error increases with time.
4.5.3.3 Ensure that the flow rate into the output manifold is at
least 1 liter/min greater than the total flow rate required by the
photometer and any other flow demand connected to the manifold.
4.5.3.4 Ensure that the flow rate of zero air, Fz, is at least 1
liter/min greater than the flow rate required by the
photometer.
4.5.3.5 With zero air flowing in the output manifold, actuate
the two-way valve to allow the photometer to sample first the
manifold zero air, then Fz. The two photometer readings must be
equal (I = I0).
Note:
In some commercially available photometers, the operation of the
two-way valve and various other operations in section 4.5.3 may be
carried out automatically by the photometer.
4.5.3.6 Adjust the O3 generator to produce an O3 concentration
as needed.
4.5.3.7 Actuate the two-way valve to allow the photometer to
sample zero air until the absorption cell is thoroughly flushed and
record the stable measured value of Io.
4.5.3.8 Actuate the two-way valve to allow the photometer to
sample the O3 concentration until the absorption cell is thoroughly
flushed and record the stable measured value of I.
4.5.3.9 Record the temperature and pressure of the sample in the
photometer absorption cell. (See Reference 13 for guidance.)
4.5.3.10 Calculate the O3 concentration from equation 4. An
average of several determinations will provide better
precision.
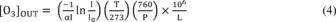
Where:
[O3]OUT = O3 concentration, ppm α = absorption coefficient of O3 at
254 nm = 308 atm−1 cm−1 at 0 °C and 760 torr l = optical path
length, cm T = sample temperature, K P = sample pressure, torr L =
correction factor for O3 losses from 4.5.2.5 = (1−fraction of O3
lost). Note:
Some commercial photometers may automatically evaluate all or
part of equation 4. It is the operator's responsibility to verify
that all of the information required for equation 4 is obtained,
either automatically by the photometer or manually. For “automatic”
photometers which evaluate the first term of equation 4 based on a
linear approximation, a manual correction may be required,
particularly at higher O3 levels. See the photometer instruction
manual and Reference 13 for guidance.
4.5.3.11 Obtain additional O3 concentration standards as
necessary by repeating steps 4.5.3.6 to 4.5.3.10 or by Option
1.
4.5.4 Certification of transfer standards. A transfer
standard is certified by relating the output of the transfer
standard to one or more O3 calibration standards as determined
according to section 4.5.3. The exact procedure varies depending on
the nature and design of the transfer standard. Consult Reference
12 for guidance.
4.5.5 Calibration of ozone analyzers. Ozone analyzers
must be calibrated as follows, using O3 standards obtained directly
according to section 4.5.3 or by means of a certified transfer
standard.
4.5.5.1 Allow sufficient time for the O3 analyzer and the
photometer or transfer standard to warm-up and stabilize.
4.5.5.2 Allow the O3 analyzer to sample zero air until a stable
response is obtained and then adjust the O3 analyzer's zero
control. Offsetting the analyzer's zero adjustment to +5% of scale
is recommended to facilitate observing negative zero drift (if
any). Record the stable zero air response as “Z”.
4.5.5.3 Generate an O3 concentration standard of approximately
80% of the desired upper range limit (URL) of the O3 analyzer.
Allow the O3 analyzer to sample this O3 concentration standard
until a stable response is obtained.
4.5.5.4 Adjust the O3 analyzer's span control to obtain the
desired response equivalent to the calculated standard
concentration. Record the O3 concentration and the corresponding
analyzer response. If substantial adjustment of the span control is
necessary, recheck the zero and span adjustments by repeating steps
4.5.5.2 to 4.5.5.4.
4.5.5.5 Generate additional O3 concentration standards (a
minimum of 5 are recommended) over the calibration scale of the O3
analyzer by adjusting the O3 source or by Option 1. For each O3
concentration standard, record the O3 concentration and the
corresponding analyzer response.
4.5.5.6 Plot the O3 analyzer responses (vertical or Y-axis)
versus the corresponding O3 standard concentrations (horizontal or
X-axis). Compute the linear regression slope and intercept and plot
the regression line to verify that no point deviates from this line
by more than 2 percent of the maximum concentration tested.
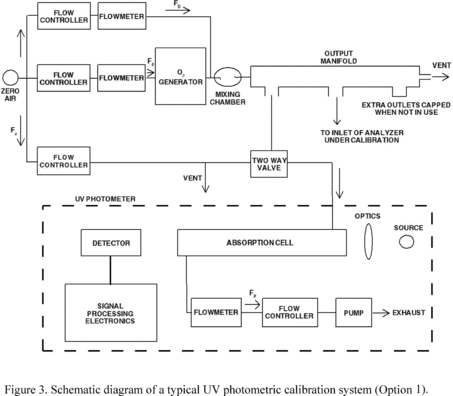
4.5.5.7 Option 1: The various O3 concentrations required
in steps 4.5.3.11 and 4.5.5.5 may be obtained by dilution of the O3
concentration generated in steps 4.5.3.6 and 4.5.5.3. With this
option, accurate flow measurements are required. The dynamic
calibration system may be modified as shown in Figure 3 to allow
for dilution air to be metered in downstream of the O3 generator. A
mixing chamber between the O3 generator and the output manifold is
also required. The flow rate through the O3 generator (Fo) and the
dilution air flow rate (FD) are measured with a flow or volume
standard that is traceable to a NIST flow or volume calibration
standard. Each O3 concentration generated by dilution is calculated
from:
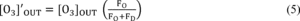
Where:
[O3]′OUT = diluted O3 concentration, ppm FO = flow rate through the
O3 generator, liter/min FD = diluent air flow rate, liter/min Note:
Additional information on calibration and pollutant standards is
provided in Section 12 of Reference 14.
5.0 Frequency of Calibration.
5.1 The frequency of calibration, as well as the number of
points necessary to establish the calibration curve, and the
frequency of other performance checking will vary by analyzer;
however, the minimum frequency, acceptance criteria, and subsequent
actions are specified in Appendix D of Reference 14: Measurement
Quality Objectives and Validation Templates. The user's quality
control program shall provide guidelines for initial establishment
of these variables and for subsequent alteration as operational
experience is accumulated. Manufacturers of analyzers should
include in their instruction/operation manuals information and
guidance as to these variables and on other matters of operation,
calibration, routine maintenance, and quality control.
6.0 References.
1. E.C.Y. Inn and Y. Tanaka, “Absorption coefficient of Ozone in
the Ultraviolet and Visible Regions”, J. Opt. Soc. Am., 43, 870
(1953). 2. A. G. Hearn, “Absorption of Ozone in the Ultraviolet and
Visible Regions of the Spectrum”, Proc. Phys. Soc. (London), 78,
932 (1961). 3. W. B. DeMore and O. Raper, “Hartley Band Extinction
Coefficients of Ozone in the Gas Phase and in Liquid Nitrogen,
Carbon Monoxide, and Argon”, J. Phys. Chem., 68, 412 (1964). 4. M.
Griggs, “Absorption Coefficients of Ozone in the Ultraviolet and
Visible Regions”, J. Chem. Phys., 49, 857 (1968). 5. K. H. Becker,
U. Schurath, and H. Seitz, “Ozone Olefin Reactions in the Gas
Phase. 1. Rate Constants and Activation Energies”, Int'l Jour. of
Chem. Kinetics, VI, 725 (1974). 6. M. A. A. Clyne and J. A. Coxom,
“Kinetic Studies of Oxy-halogen Radical Systems”, Proc. Roy. Soc.,
A303, 207 (1968). 7. J. W. Simons, R. J. Paur, H. A. Webster, and
E. J. Bair, “Ozone Ultraviolet Photolysis. VI. The Ultraviolet
Spectrum”, J. Chem. Phys., 59, 1203 (1973). 8. Ollison, W.M.; Crow,
W.; Spicer, C.W. “Field testing of new-technology ambient air ozone
monitors.” J. Air Waste Manage. Assoc., 63 (7), 855-863 (2013). 9.
Parrish, D.D.; Fehsenfeld, F.C. “Methods for gas-phase measurements
of ozone, ozone precursors and aerosol precursors.” Atmos.
Environ., 34 (12-14), 1921-1957(2000). 10. Ridley, B.A.; Grahek,
F.E.; Walega, J.G. “A small, high-sensitivity, medium-response
ozone detector suitable for measurements from light aircraft.” J.
Atmos. Oceanic Technol., 9 (2), 142-148(1992). 11. Boylan, P.,
Helmig, D., and Park, J.H. “Characterization and mitigation of
water vapor effects in the measurement of ozone by
chemiluminescence with nitric oxide.” Atmos. Meas. Tech. 7,
1231-1244 (2014). 12. Transfer Standards for Calibration of Ambient
Air Monitoring Analyzers for Ozone, EPA publication number
EPA-454/B-13-004, October 2013. EPA, Office of Air Quality Planning
and Standards, Research Triangle Park, NC 27711. [Available at
www.epa.gov/ttnamti1/files/ambient/qaqc/OzoneTransferStandardGuidance.pdf.]
13. Technical Assistance Document for the Calibration of Ambient
Ozone Monitors, EPA publication number EPA-600/4-79-057, September,
1979. [Available at
www.epa.gov/ttnamti1/files/ambient/criteria/4-79-057.pdf.]
14. QA Handbook for Air Pollution Measurement Systems - Volume II.
Ambient Air Quality Monitoring Program. EPA-454/B-13-003, May 2013.
[Available at
http://www.epa.gov/ttnamti1/files/ambient/pm25/qa/QA-Handbook-Vol-II.pdf.]
[80 FR 65453, Oct.
26, 2015]