Title 40
PART 300 APPENDIX C
| Major Ion | % Total Weight | Ionic Concentration at 34 ppt salinity (mg/1) |
|---|---|---|
| Chloride (C1−) | 47.470 | 18,740 |
| Sodium (NA=) | 26.280 | 10,454 |
| Sulfate (SO4−) | 6.602 | 2,631 |
| Magnesium (Mg==) | 3.230 | 1,256 |
| Calcium (Ca==) | 1.013 | 400 |
| Potassium (K=) | 1.015 | 401 |
| Bicarbonate (HCO3−) | 0.491 | 194 |
| Boron (B) | 0.015 | 6.0 |
| Strontium (Sr==) | 0.001 | 7.5 |
| SOLIDS TOTAL | 86.11% | 34,089.50 |
| Water | 13.88 | |
| TOTAL | 99.99% |
2.3.2 Test oil. Two EPA/American Petroleum Institute (API) standard reference oils, Prudhoe Bay and South Louisiana crude, should be used for this test. These oils can be obtained from the Resource Technology Corporation, 2931 Soldier Springs Road, P.O. Box 1346, Laramie, WY 82070, (307) 742-5452. These oils have been thoroughly homogenized, as well as characterized physically and chemically for previous EPA and API studies. Various selected parameters are presented in table 2.
Table 2 - Test Oil Characteristics
| Prudhoe Bay crude oil | South Louisiana crude oil | |
|---|---|---|
| Specific gravity 1 | 0.894 kg/1 | 0.840 kg/1 |
| API gravity 1 | 26.8 degrees | 37.0 degrees |
| Sulfur | 1.03 wt% | 0.23 wt% |
| Sulfur compounds, profile | ||
| Nitrogen | 0.20 wt% | 0.031 wt% |
| Vanadium | 21 mg/1 | 0.95 mg/1 |
| Nickel | 11 mg/1 | 1.1 mg/1 |
| Simulated distillation profile | ||
| Infrared spectrum | ||
| UV fluorescence spectrum | ||
| Pour Point | + 25 °F | 0 °F |
| Viscosity | ||
| at 40 °C | 14.09 cST | 3.582 cST |
| at 100 °C | 4.059 cST | 1.568 cST |
| Index | 210 | ( 2) |
1 At 15 °C
2 Not calculable when viscosity at 100 °C is less than 2.0.
2.3.3 Methylene Chloride (Dichloromethane-DCM), pesticide quality. For extraction of all sample water and oil-standard water samples.
2.4 Pretest preparation. 2.4.1 Preparation and analysis of oil standards. 2.4.1.1 Standard solutions of oil for calibrating the UV-visible spectrophotometer are prepared with the specific reference oils and dispersant used for a particular set of experimental test runs. For experiments with no dispersant, only oil is used to make the standard solution. For experiments with the oil plus dispersant, the standard is made with a 1:10 (v:v) mixture of the dispersant to the test oil (i.e., a dispersant-to-oil ratio of 1:10). This ratio is used in the test tank with dispersant added. The presence of water and certain dispersants in DCM extracts can affect absorbance readings in a spectrophotometer. All standard solutions of oil (and dispersant, if present) should be prepared in a stepwise manner that reflects the analytical protocol used for the experimental water samples.
2.4.1.2 To prepare the standards, prepare a parent oil-DCM standard by mixing 1 part oil (plus 1/10 part premixed dispersant, if applicable) to 9 parts DCM (i.e., 1:10 dilution of the oil v:v). Add a specific volume of the parent oil-DCM standard to 30 ml of synthetic seawater in a separatory funnel. Extract the oil-water mixture with 5-ml volumes of DCM after 15 seconds of vigorous shaking followed by a 2 minute stationary period to allow for phase separation for each extraction. Repeat the extraction using a total of three 5-ml portions of DCM. Adjust the final DCM volume for the combined extracts to 20 ml with DCM in a 25-ml graduated cylinder.
2.4.1.3 The quantities of oil used to achieve the desired concentrations in the final 20-ml DCM extracts for the standard oil-solutions are summarized in table 3. Specific masses for oil amounts in standards are determined as volumes of oil multiplied by the density of the oil.
2.4.2 Linear stability calibration of UV-Visible spectrophotometer.
2.4.2.1 Before DCM-extracts of dispersed oil-water samples can be analyzed for their oil content, the UV-visible spectrophotometer must meet an instrument stability calibration criterion. This criterion is determined with the six oil standards identified in table 3. Determine the absorbance of standards at each of the three analytical wavelengths (i.e., 340, 370, and 400 nm). Determine the response factors (RFs) for the test oil at each of the three analytical wavelengths using the following equation:
RFx = C/Ax (1) where: RFx = Response factor at wavelength x (x = 340, 370, or 400 nm) C = Oil concentration, in mg of oil/ml of DCM in standard solution Ax = Spectrophotometric absorbance of wavelength xTable 3 - Oil Standard Solutions: Concentrations in Final DCM Extractions 1
| Final oil concentration (mg/ml of DCM) |
Final extract volume (ml of DCM) |
Total amount of oil in standard (mg) |
Volume of parent oil-DCM std (µl) added to saltwater |
|---|---|---|---|
| 4.0 | 20.0 | 80.0 | 890 |
| 2.0 | 20.0 | 40.0 | 440 |
| 1.0 | 20.0 | 20.0 | 220 |
| 0.50 | 20.0 | 10.0 | 110 |
| 0.10 | 20.0 | 2.0 | 22 |
| 0.05 | 20.0 | 1.0 | 11 |
1 Assuming an oil density of 0.9 g/ml and an extraction efficiency of 100% for oil from the 30-ml of seawater.
2.4.2.2 Instrument stability for the initial calibration is acceptable when the RFs for the five highest standard extracts of oil are <20% different from the overall mean value for the five standards. If this criterion is satisfied, analysis of sample extracts can begin. RFs for the lowest concentration (0.05 mg oil/ml DCM) are not included in the consideration because the absorbance is close to the detection limit of the spectrophotometer (with associated high variability in the value) for the 1-cm path-length cell used for measurements. Absorbances ≥3.5 are not included because absorbance saturation occurs at and above this value.
2.4.2.3 If one or more of the standard oil extracts do not meet this linear-stability criterion, then the “offending” standard(s) can be prepared a second time (i.e., extraction of the specified amount of oil from 30-ml or seawater for the “offending” standard according to the pretest preparation procedure). If replacement of the reanalyzed standard solution(s) in the standard curve meets the linear-stability criterion (i.e., no RF >20% different from the overall mean), then analysis of sample extracts can begin.
2.4.2.4 If the initial-stability criterion is still not satisfied, analysis of sample extract cannot begin and the source of the problem (e.g., preparation protocol for the oil standards, spectrophotometer stability, etc.) must be corrected.
2.4.2.5 The initial six-point calibration of the UV-visible spectrophotometer at the oil concentrations identified is required at least once per test day.
2.5 Test procedure. 2.5.1 Preparation of premixed dispersant oil. Prepare a premixed dispersant oil by mixing 1 part dispersant to 10 parts oil. Store this mixture in a glass container. The dispersant effectiveness test procedures are listed in steps 1-20:
1. Prepare 4 replicates (same test oil and dispersant), one control (i.e., no dispersant), and one method blank and run at the same time on the shaker table.
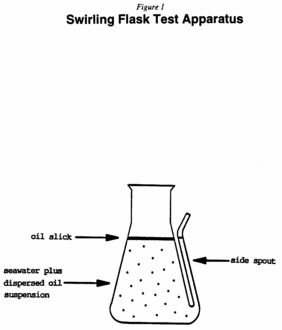
2. Add 120±2 ml of synthetic seawater to each of the modified 125-ml glass Erlenmeyer flasks. Measure and record the water temperature.
3. Place the flasks securely into the attached slot on the shaker table.
4. Carefully add 100 µl of an oil-dispersant solution onto the center of the water's surface using a positive displacement pipette.
5. Agitate the flasks for 20±1 minutes at 150±10 rpm on the shaker table.
6. After the 20±1 minutes shaking, remove the flasks from the shaker table and allow them to remain stationary for 10±1 minutes for oil droplet “settling.”
7. At the conclusion of the 10-minute settling period, carefully decant a 30-ml sample through the side spout of the test flasks into a 50-ml graduated cylinder.
Note:Discard the first 1-2 ml of sample water to remove nonhomogeneous water-oil initially contained in the spout.
8. Transfer the samples from the graduated cylinder into a 125- or 250-ml glass separatory funnel fitted with a Teflon stopcock.
9. Add 5 ml of pesticide-quality DCM to the separatory funnel and shake vigorously for 15 seconds. Release the pressure carefully from the separatory funnel through the stopcock into a fume hood.
10. Allow the funnel to remain in a stationary position for 2 minutes to allow phase-separation of the water and DCM.
11. Drain the DCM layer from the separatory funnel into a glass-stoppered, 25-ml graduated glass cylinder.
12. Repeat the DCM-extraction process two additional times.
13. Combine the three extracts in the graduated cylinder and adjust the final volume to 20-ml with additional DCM.
14. Analyze the samples using a UV-spectrophotometer at 340, 370, and 400 nm-wavelengths and determine the quantity of oil as follows:
Cx = (Ax)x(RFx)x(VDCM)x(Vtw/Vew) (2) where: Cx = Total mass of dispersed oil in swirling flask at wavelength x (x = 340, 370, or 400 nm) Ax = Spectrophotometric absorbance at wavelength x RFx = Mean response factor at wavelength x (determined from equation 1) VDCM = Final volume of DCM-extract of water sample (20 ml) Vtw = Total water volume in swirling flask vessel (120 ml) Vew = Volume of water extracted for dispersed oil content (30 ml)15. Obtain three concentration values for oil in each experimental water sample (340, 370, and 400 nm).
16. Determine the mean of three values as follows:
Cmean = (C340 + C370 + C400)/3 (3) Note:Means will be used for all dispersion-performance calculations. Samples where one of the values for C340, C370, or C400 is more than 30% different from Cmean will be flagged. Whenever oil measurements are flagged as having a concentration based on one wavelength as >30% different from Cmean, raw data will be evaluated to establish that the measurements are valid. In addition, attempts will be made to correlate the difference to oil type, dispersant test, or dispersant used. If no errors or correlations are apparent and >10% of all oil measurements are flagged, the mean concentration data will be used in the calculation for dispersant performance and the subject data will be flagged.
17. Determine the dispersant performance (i.e., percent of oil that is dispersed, or EFF) based on the ratio of oil dispersed in the test system to the total oil added to the system as follows:
EFF (in %) = (Cmean/CTOT) × 100 (4) where: Cmean = Mean value for total mass of dispersed oil in the swirling flask determined by spectrophotometric analysis CTOT = Total mass of oil initially added to the experimental swirling flask18. Calculate EFF using equation 4 for coupled experiments with and without dispersant (EFFc and EFFd, respectively). EFFc is the effectiveness of the control and represents natural dispersion of the oil in the test apparatus. EFFd is the measured uncorrected value.
19. Calculate the final dispersant performance of a chemical dispersant agent after correcting for natural dispersion using equation 5.
EFFD = EFFd - EFFc (5) where: EFFD = % dispersed oil due to dispersant only EFFd = % dispersed oil with dispersant added EFFc = % dispersed oil with no dispersant added20. Calculate the average dispersant effectiveness value by summing the corrected values (EFFD) for each of the four replicates for each of the two test oils and dividing this sum by eight.
2.6 Performance criterion. The dispersant product tested will remain in consideration for addition to the NCP Product Schedule if the average dispersant effectiveness, as calculated in section 2.5 above, is at least 45% (i.e., 50%±5%).
2.7 Quality Control (QC) procedures for measurements of oil concentrations. 2.7.1 UV-visible spectrophotometric measurements. At least 5% of all UV-visible spectrophotometric measurements will be performed in duplicate as a QC check on the analytical measurement method. The absorbance values for the duplicates should agree within ±5% of their mean value.
2.7.2 Method blanks. Analytical method blanks involve an analysis of seawater blanks (i.e., seawater but no oil or dispersant in a swirling flask vessel) through testing and analytical procedures (3, pp 79-80). Method blanks are analyzed with a frequency of at least 1 for every 12 experimental swirling flask samples. Oil concentrations in method blanks must be <5% of that occurring for 100% dispersion of oil in testing apparatus.
3.0 Revised standard dispersant toxicity test3.1 Summary of method. The standard toxicity test for dispersants and other products involves exposing two species (Menidia beryllina (silversides) and Mysidopsis bahia (mysid shrimp)) to five concentrations of the test product and No. 2 fuel oil alone and in a 1:10 mixture of product to oil. To aid in comparing results from assays performed by different workers, reference toxicity tests are conducted using dodecyl sodium sulfate (DSS) as a reference toxicant. The test length is 96 hours for Menidia and 48 hours for Mysidopsis. LC50 s are calculated based on mortality data at the end of the exposure period (for method of calculation, see section 3.6 below).
3.2 Selection and preparation of test materials.
3.2.1 Test organisms.
3.2.1.1 Menidia beryllina. Obtain fish (silversides) from a single source for each series of toxicity tests. In-house cultures are recommended wherever it is cost-effective; however, organisms are available from commercial suppliers. Information on the source of test organisms and any known unusual condition to which fish were exposed before use should be included in the data report. Use of animals previously treated with pesticides or chemotherapeutic agents should be avoided. Organisms should not be used if they appear to be unhealthy, discolored, or show signs of stress. Use 7-day old larval fish. Fish should be cultured in accordance with the methods outlined in Middaugh, et al. (5). There should be no need to acclimate organisms to the 25±1 °C temperature recommended for the toxicity tests if laboratory stock cultures of Menidia are maintained at the recommended culture temperature of 25±1 °C. If test organisms must be obtained from a commercial source, it may become necessary to acclimate test fish to the test temperature of 25±1 °C, a pH of 8.0±0.2, and 20±2 ppt salinity since changes in temperature may occur during shipping. Eliminate groups of fish having a mortality of more than 10% during the first 48 hours, and more than 5% thereafter. During acclimation, organisms should be maintained on a diet of freshly hatched Artemia (brine shrimp) nauplii. Feed the fish daily to satiation during the acclimation period, and once daily during the 96-hour test. Care should be taken daily to remove excess food and fecal material from beakers during the test. Use only those organisms that feed actively and that appear to be healthy. Organisms should be free of disease, external parasites, and any signs of physical damage or stress. Discard any fish injured or dropped while handling.
3.2.1.2 Mysidopsis bahia. Several methods for culturing Mysidopsis bahia (mysid shrimp) may be used and are noted in appendix A of Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms (6). To ensure uniformity of mysids, recently hatched mysids should be collected daily from stock cultures and identified by the date of hatch. Mysids used in 48-hour tests should be from a single day's collection, but may have an age range of 5-7 days old. In cases where in-house cultures of mysids are unavailable, organisms may be purchased from a commercial source. Information on the source of test organisms should be submitted in the data report.
3.2.2 Preparation of experimental water. Filtered natural seawater is recommended for use since it represents a natural source of saltwater containing an inherent population of microorganisms. Synthetic seawater formulated according to the following method can serve as an acceptable alternative to filtered, natural seawater for toxicity tests performed in laboratories in which natural seawater is unavailable.
3.2.3 Synthetic seawater formation. To prepare standard seawater, mix technical-grade salts with 900 liters of distilled or demineralized water in the order and quantities listed in table 4. These ingredients must be added in the order listed and each ingredient must be dissolved before another is added. Stir constantly after each addition during preparation until dissolution is complete. Add distilled or demineralized water to make up to 1,000 liters. The pH should now be 8.0±0.2. To attain the desired salinity of 20±1 ppt, dilute again with distilled or demineralized water at time of use.
3.3 Sampling and storage of test materials. Toxicity tests are performed with No. 2 fuel oil having the characteristics defined in table 5. Store oil used for toxicity tests in sealed containers to prevent the loss of volatiles and other changes. For ease in handling and use, it is recommended that 1,000-ml glass containers be used. To ensure comparable results in the bioassay tests, use oils packaged and sealed at the source. Dispose of unused oil in each open container on completion of dosing to prevent its use at a later date when it may have lost some of its volatile components. Run all tests in a bioassay series with oil from the same container and with organisms from the same group collected or secured from the same source.
Table 4 - Synthetic Seawater
[Toxicity Test]
| Salt | (g) 1 |
|---|---|
| NaF | 1.9 |
| SrCl2 · 6H2O | 13.0 |
| H3 BO2 | 20.0 |
| KBr | 67.0 |
| KCl | 466.0 |
| CaC12 · 2H2O | 733.0 |
| Na2 SO4 | 2,660.0 |
| MgCl2 · 6H2O | 3,330.0 |
| NaCl | 15,650.0 |
| Na2SiO3 · 9H2O | 13.0 |
| EDTA 2 | 0.4 |
| NaHCO3 | 133.0 |
1 Amount added to 900 liters of water, as described in the text.
2 Ethylenediaminetetraacetate tetrasodium salt.
3.4 General test conditions and procedures for toxicity tests.
3.4.1 Temperature. For these toxicity tests, use test solutions with temperatures of 25±1 °C.
3.4.2 Dissolved oxygen and aeration.
3.4.2.1 Menidia. Because oils contain toxic, volatile materials, and because the toxicity of some water-soluble fractions of oil and degradation products are changed by oxidation, special care must be used in the oxygenation of test solutions. Aeration during the test is generally not recommended but should be used to maintain the required dissolved oxygen (DO) in cases where low DO is observed. The DO content of test solutions must not drop below 60% saturation during the first 48 hours of a static acute (96-hour) test and must remain between 40-100% after the first 48 hours of the test. Aeration at a rate of 100±15 bubbles per minute is supplied by a serological pipette as needed for maintenance of DO. If aeration is necessary, all test chambers should be aerated. At this rate, and with the proper weight of fish, DO concentration should remain slightly above 4 ppm over a 96-hour period. Take DO measurements daily.
Table 5 - Test Oil Characteristics: No. 2 Fuel Oil
| Characteristic | Minimum | Maximum |
|---|---|---|
| Gravity (°API) | 32.1 | 42.8 |
| Viscosity kinematic at 100 °F (cs) | 2.35 | 3.00 |
| Flash point (°F) | 150 | ... |
| Pour point (°F) | ... | 0 |
| Cloud point (°F) | ... | 10 |
| Sulfur (wt %) | ... | 0.35 |
| Aniline point (°F) | 125 | 180 |
| Carbon residue (wt %) | ... | 0.16 |
| Water (vol %) | ... | 0 |
| Sediment (wt %) | ... | 0 |
| Aromatics (vol %) | 10 | 15 |
| Distillation: | ||
| IBP (°F) | 347 | 407 |
| 10% (°F) | 402 | 456 |
| 50% (°F) | 475 | 530 |
| 90% (°F) | 542 | 606 |
| End Point (°F) | 596 | 655 |
| Neutralization No | ... | 0.05 |
3.4.2.2 Mysidopsis. Achieve sufficient DO by ensuring that the surface area to volume ratio of the test solution exposed is large enough. Oxygen content should remain high throughout the test because of the low oxygen demand of the organisms. Aeration is not recommended during 48-hour acute toxicity tests unless the DO falls below 60% saturation.
3.4.3 Controls. With each fish or mysid test or each series of simultaneous tests of different solutions, perform a concurrent control test in exactly the same manner as the other tests and under the conditions prescribed or selected for those tests. Use the diluent water alone as the medium in which the controls are held. There must be no more than 10% mortality among the controls during the course of any valid test.
3.4.4 Reference toxicant. To aid in comparing results from tests performed by different workers and to detect changes in the condition of the test organisms that might lead to different results, perform reference toxicity tests with reagent grade DSS in addition to the usual control tests. Prepare a stock solution of DSS immediately before use by adding 1 gram of DSS per 500 ml of test water solution. Use exploratory tests before the full scale tests are begun to determine the amount of reference standard to be used in each of the five different concentrations.
3.4.5 Number of organisms. At a minimum, 20 organisms of a given species are exposed for each test concentration. For the toxicity test procedures using Menidia, place 10 fish in each of two jars. For the toxicity tests using Mysidopsis, place 10 larvae in each of two containers.
3.4.6 Transfer of organisms. Organisms should be handled as little as possible in order to minimize stress. Transfer Menidia and Mysidopsis from the acclimatization aquaria to the test chambers with a pipette or a wide-bore, smooth glass tube (4 to 8 mm internal diameter) fitted with a rubber bulb. Dip nets should be avoided when handling larval fish and mysids. Do not hold fish out of the water longer than necessary and discard any specimen accidentally dropped or otherwise mishandled during transfer.
3.4.6.1 Mysidopsis. To have the mysids ready for study, mysids may be sorted 24 hours prior to initiation of the 48-hour test. Transfer the mysids to a beaker containing a small volume of water; this vessel serves as a holding chamber during randomized transfer of the organisms to test solutions. Mysids are randomly selected from the batch of mysids in the holding chamber, and transferred to 50-ml beakers containing a small volume of seawater. One mysid is added per beaker using a small piece of flexible 500-µm screening until all of the beakers contain one mysid. The process of random selection and sorting is continued until the appropriate number of mysids has been delivered to each of the 50-ml beakers. The mysids are gently released from the 50-ml beakers into larger beakers filled with an appropriate volume of 20-ppt seawater (25 °C) to bring the total volume to 200 ml. The beakers are randomly placed into a temperature-controlled water bath to acclimate overnight at 25 °C. The mysids are transferred to larger beakers (1-liter) for the 48-hour test after the addition of 800 ml of the test solution. A total of 10 mysids per beaker are used for 48-hour acute toxicity tests. A minimum of two replicate chambers are used for each test concentration and control.
3.4.6.2 Menidia and Mysidopsis are fed 50 brine shrimp nauplii/organism daily during the 96-hour and 48-hour tests. Excess food should be removed daily by aspirating with a pipette.
3.4.7 Test duration and observations. 3.4.7.1 Menidia. Observe the number of dead fish in each test container and record at the end of each 24-hour period. Fish are considered dead upon cessation of respiratory and all other overt movements, whether spontaneous or in response to mild mechanical prodding. Remove dead fish as soon as observed. Also note and report when the behavior of test fish deviates from that of control fish. Such behavioral changes would include variations in opercular movement, coloration, body orientation, movement, depth in container, schooling tendencies, and others. Abnormal behavior of the test organisms (especially during the first 24 hours) is a desirable parameter to monitor in a toxicity test because changes in behavior and appearance may precede mortality. Toxicants can reduce an organism's ability to survive natural stresses. In these cases, the mortality is not directly attributed to the toxicant, but most certainly is an indirect effect. Reports on behavioral changes during a toxicity test can give insight into the non-acute effects of the tested material. At the end of the 96-hour period, terminate the fish tests and determine the LC50 values. The acute toxicity test is terminated after four days of exposure. The number of surviving fish are counted and recorded for each chamber in accordance with standard EPA methods (6). The LC50 is calculated using survival data from the test in accordance with the methods described in the guidelines (6).
3.4.7.2 Mysidopsis. Terminate the mysid test after 48 hours of incubation. To count the dead animals accurately, place the exposure vessels on a light table such that light passes through the bottom of the vessel. Most of the dead mysids will be on the bottom of the beaker and can readily be seen against the background of the light table. Also search the top of the liquid for mysids trapped there by surface tension. Exercise caution when determining death of the animals. Occasionally, an animal appears dead, but closer observation shows slight movement of an appendage or a periodic spasm of its entire body. For these tests, animals exhibiting any movement when touched with a pipette tip are considered alive. Account for all test animals to ensure accuracy since Mysidopsis bahia may disintegrate or be cannibalized by other mysids. Consider individuals not accounted for as dead. At the end of 48 hours of exposure, terminate the mysid assay and determine the LC50 values in accordance with the methods described in the guidelines (6).
3.4.8 Physical and chemical determinations. 3.4.8.1 Menidia. Determine the temperature, DO, and pH of the test solutions before the fish are added and at 24-, 48-, 72-, and 96-hour exposure intervals. It is necessary to take measurements from only one of the replicates of each of the toxicant series.
3.4.8.2 Mysidopsis. Determine the temperature, DO, and pH of the test solutions before the nauplii are added and at the 24- and 48-hour exposure interval. Measure DO and pH in only one of the replicates of each of the toxicant series.
3.4.9 Testing laboratory. An ordinary heated or air-conditioned laboratory room with thermostatic controls suitable for maintaining the prescribed test temperatures generally will suffice to conduct the toxicity tests. Where ambient temperatures cannot be controlled to 25±1 °C, use water baths with the necessary temperature controls.
3.4.10 Test containers. For tests with fish or mysids, use 1-liter glass beakers measuring approximately 10 cm in diameter. In conducting the test, add to each beaker 1 liter of the test solution or seawater formulation aerated to saturation with DO. To add the liter volume easily and accurately, use a large volume (1-liter) graduated cylinder. Process all required glassware before each test. Immerse in normal hexane for 10 minutes. Follow this with a thorough rinse with hot tap water; three hot detergent scrubs; an additional hot tap-water rinse; and three rinses with distilled water. Oven or air dry the glassware in a reasonably dust-free atmosphere.
3.5 Preparation of test concentrations. 3.5.1 Menidia. Place test jars (approximately 22.5 cm in height, 15 cm in diameter, 11 cm in diameter at the mouth) containing 2 liters of synthetic seawater on a reciprocal shaker. The shaker platform should be adapted to hold firmly six of the toxicity test jars. Add the desired amount of the petroleum product (if applicable) under test directly to each test jar. Dispense the appropriate amount of toxicant (if applicable) into the jars with a pipette. Tightly cap the test jars and shake for 5 minutes at approximately 315 to 333 2-cm (0.75-inch) strokes per minute in a reciprocal shaker or at approximately 150 to 160 rpm on orbital shakers. At the completion of shaking, remove the jars from the shaker and dispense 1 liter of the mixture to each of the 1-liter glass beakers. Randomly place beakers in a constant-temperature water bath or room, take water quality measurements, add fish, and initiate aeration.
3.5.2 Mysidopsis. 3.5.2.1 To prepare test solutions for products and oil/product mixtures, blend or mix the test solutions with an electric blender having: speeds of 10,000 rpm or less; a stainless-steel cutting assembly; and a 1-liter borosilicate jar. To minimize foaming, blend at speeds below 10,000 rpm.
3.5.2.2 For the product test solution, add 550 ml of the synthetic seawater to the jar, then with the use of a gas-tight calibrated glass syringe with a Teflon-tipped plunger, add 0.55 ml of the product and mix for 5 seconds.
3.5.2.3 For the oil test solution, add 550 ml of the synthetic seawater to the jar. Then with the use of a gas-tight calibrated glass syringe equipped with a Teflon-tipped plunger, add 0.55 ml of the oil and mix for 5 seconds.
3.5.2.4 For the oil/product mixture, add 550 ml of the synthetic seawater to the mixing jar. While the blender is in operation, add 0.5 ml of the oil under study with the use of a calibrated syringe with a Teflon-tipper plunger and then 0.05 ml of the product as indicated above. Blend for 5 seconds after addition of product. These additions provide test solutions of the product, oil, and the oil/product mixture at concentrations of 1,000 ppm.
3.5.2.5 Immediately after the test solutions are prepared, draw up the necessary amount of test solution with a gas-tight Teflon-tipped glass syringe of appropriate size and dispense into each of the five containers in each series. If the series of five concentrations to be tested are 10, 18, 32, 56, and 100 ppm, the amount of the test solution in the order of the concentrations listed above would be as follows: 10, 18, 32, 56, and 100 ml.
3.5.2.6 Each time a syringe is to be filled for dispensing to the series of test containers, start the mixer and withdraw the desired amount in the appropriate syringe while the mixer is in operation. Turn off immediately after the sample is taken to limit the loss of volatiles.
3.5.2.7 Use exploratory tests before the full-scale test is set up to determine the concentration of toxicant to be used in each of the five different concentrations. After adding the required amounts of liquid, bring the volume in each of the test containers up to 800 ml with the artificial seawater. To ensure keeping each of the series separate, designate on the lid of each container the date, the material under test, and its concentration.
3.5.2.8 When the desired concentrations are prepared, gently release into each beaker the 10 test Mysidopsis (previously transferred into 200 ml of medium). This provides a volume of 1 liter in each test chamber. A pair of standard cover glass forceps with flat, bent ends is an ideal tool for handling and tipping the small beaker without risk of contaminating the medium.
3.5.2.9 After adding the test animals, incubate the test beakers at 25±1 °C for 48 hours. Recommended lighting is 2,000 lumens/m 2 (200 ft-c) of diffused, constant, fluorescent illumination.
3.5.2.10 Wash the blender thoroughly after use and repeat the above procedures for each series of tests. Wash the blender as follows: rinse with normal hexane; pour a strong solution of laboratory detergent into the blender to cover the blades; fill the container to about half of its volume with hot tap water; operate the blender for about 30 seconds at high speed; remove and rinse twice with hot tap water, mixing each rinse for 5 seconds at high speed; and then rinse twice with distilled water, mixing each rinse for 5 seconds at high speed.
3.6 Calculating and reporting. At the end of the test period, the toxicity tests are terminated and the LC50 values are determined.
3.6.1 Calculations. The LC50 is the concentration lethal to 50% of the test population. It can be calculated as an interpolated value based on percentages of organisms surviving at two or more concentrations, at which less than half and more than half survived. The LC50 can be estimated with the aid of computer programs or graphic techniques (log paper). The 95% confidence intervals for the LC50 estimate should also be determined.
3.6.2 Reporting. The test product and oil and their source and storage are described in the toxicity test report. Note any observed changes in the experimental water or the test solutions. Also include the species of fish used; the sources, size, and condition of the fish; data of any known treatment of the fish for disease or infestation with parasites before their use; and any observations on the fish behavior at regular intervals during the tests. In addition to the calculated LC50 values, other data necessary for interpretation (e.g., DO, pH, other physical parameters, and the percent survival at the end of each day of exposure at each concentration of toxicant) should be reported.
3.7 Summary of procedures. 3.7.1 Menidia:
1. Prepare adequate stocks of the appropriate standard dilution water.
2. Add 2 liters of the standard dilution water to the test jars. Each test consists of 5 replicates of each of 5 concentrations of the test material, a control series of 5 beakers, and a standard reference series of 5 different concentrations for a total of 35 beakers. Simultaneous performance of toxicity tests on the oil, product, and oil/product mixture requires a total of 105 beakers.
3. Add the determined amount (quarter points on the log scale) of test material to the appropriate jars. Preliminary tests will be necessary to define the range of definitive test concentrations.
4. Cap the jars tightly with the Teflon-lined screw caps and shake for 5 minutes at 315 to 333 2-cm (0.75-inch) strokes per minute on a reciprocal shaker.
5. Remove the jars from the shaker, take water quality data, dispense 1 liter of solution to the 1-liter glass beaker, and add 10 acclimated fish per beaker.
6. Aerate with 100±15 bubbles per minute through a 1-ml serological pipette, as needed, to maintain DO above 4.0 mg/l.
7. Observe and record mortalities, water quality, and behavioral changes every 24 hours.
8. After 96 hours, terminate the test, and calculate LC50 values and corresponding confidence limits.
3.7.2 Mysidopsis:
1. Initiate the procedure for hatching the Mysidopsis in sufficient time before the toxicity test is to be conducted so that 5-7 day old larvae are available.
2. With the use of a small pipette, transfer 10 Mysidopsis into small beakers, each containing 200 ml of the proper synthetic seawater.
3. To prepare the test stock product and oil solutions, add 550 ml of the artificial seawater to the prescribed blender jar. By means of a gas-tight glass syringe with a Teflon-tipped plunger, add 0.55 ml of the product (or oil) and mix at 10,000 rpm for 5 seconds. To prepare the test stock oil/product mixture, add 550 ml of the standard seawater to the blender jar. While the blender is in operation (10,000 rpm), add 0.5 ml of the oil, then 0.05 ml of the product with the use of a calibrated syringe with a Teflon-tipped plunger. Blend for 5 seconds after adding the product. One ml of these stock solutions added to the 100 ml of standard seawater in the test containers yields a concentration of 10 ppm product, oil, or oil/product combination (the test will be in a ratio of 1 part product to 10 parts of oil).
4. Each test consists of 5 replications of each of 5 concentrations of the material under study, a control series of 5 beakers and a standard reference series of 5 different concentrations, for a total of 35 beakers. Simultaneous performance of toxicity tests on the oil, product, and oil/product mixture requires a total of 105 beakers. Immediately after preparing the test solution of the product or oil/product solution, and using an appropriately sized syringe, draw up the necessary amount of test solution and dispense into each of the five containers in each series. Each time a syringe is to be filled for dispensing to the series of test containers, start the mixer and withdraw the desired amount in the appropriate syringe while the mixer is in operation. Turn mixer off immediately after the sample is taken to limit the loss of volatiles. After adding the required amount of the test oil/product or product mixture, bring the volume of liquid in each of the test containers up to 800 ml with the artificial seawater. When the desired concentrations have been prepared, gently release into each beaker the 10 mysids previously transferred into 200 ml of medium. This provides a volume of 1 liter in each test chamber.
5. Wash the blender as prescribed for each series of tests.
6. Incubate the test beakers at 25±1 °C for 48 hours with the prescribed lighting.
7. Terminate the experiment after 48 hours, observe and record the mortalities, and determine the LC50 s and corresponding confidence limits.
4.0 Bioremediation agent effectiveness test4.1 Summary of method. The bioremediation agent effectiveness testing protocol is designed to determine a product's ability to biodegrade oil by quantifying changes in the oil composition resulting from biodegradation. The protocol tests for microbial activity and quantifies the disappearance of saturated hydrocarbons and polynuclear aromatic hydrocarbons (PAHs). The sample preparation procedure extracts the oil phase into dichloromethane (DCM), with a subsequent solvent exchange into hexane. To effectively accomplish the goals of the testing protocol, it is necessary to normalize the concentration of the various analytes in oil to a non-biodegradable marker, either C2-or C3-phenanthrene, C2-chrysene, or hopane 1 (7). The test method targets the relatively easy to degrade normal alkanes and the more resistant and toxic PAHs. It normalizes their concentrations to C2-or C3-phenanthrene, C2-chrysene, or C3017α(H), 21β (H)-hopane on an oil weight basis (mg marker/kg oil, mg target analyte/kg oil). The analytical technique uses a high resolution gas chromatograph/mass spectrometer (GC/MS) because of its high degree of chemical separation and spectral resolution. GC/MS has long been used to study the weathering and fate of oil spilled into the environment. For quantitative analyses, the instrument is operated in the selective ion detection (SIM) mode at a scan rate of greater than 1.5 scans per second to maximize the linear quantitative range and precision of the instrument. The sample preparation method does not exclude analysis of selected samples by GC/MS in the full scanning mode of operation to qualitatively assess changes in the oil not accounted for by the SIM approach. Performed concurrently with the chemical analysis described above is a microbiological analysis. The microbiological analysis is performed to determine and monitor the viability of the microbial cultures being studied. Under this procedure, microbial enumerations of hydrocarbon degraders are performed at each sampling event using a microtiter Most Probable Number (MPN) determination.
1 Although any of these biomarkers can be used to conduct this test, it is recommended that hopane be used.
4.2 Apparatus. The following materials and equipment are required for the protocol: Appropriate flasks and other glassware; sterile tubes; graduated cylinders (100-ml); deionized water; p-iodonitrotetrazolium violet dye; weighing pans or paper; 250-ml borosilicate glass Erlenmeyer flasks with screw tops; Pasteur pipettes; laboratory notebook; microtiter MPN plates (24-well) multi-channel pipetting device; dilution tube and caps; autoclave; environmental room or incubator; balance accurate to 0.1 mg (XD-400); GC/MS instrument equipped with a DB-5 capillary column (30 m, 0.25-mm I.D., and 0.25-µm film thickness) and a split/splitless injection port operating in the splitless mode, such as Hewlett-Packard 5890/5971 GC/MS (recommended for use); and an autosampler for testing multiple samples.
4.3 Reagents and culture medium. 4.3.1 Preparation of seawater. All products are tested in clean natural seawater. Clean natural seawater means that the source of this seawater must not be heavily contaminated with industrial or other types of effluent. For example, seawater should not be obtained from a source near shipping channels or discharges of industrial or municipal wastewater, or with high turbidity. The seawater is used within seven days of collection. No microbial inoculum is added.
4.3.2 Preparation of oil. A medium weight crude oil, Alaska North Slope (ANS), is artificially weathered by heating to 521 °F to remove the light end hydrocarbons prior to experimental start-up (ANS 521). The method is described in the Draft International Standard ISO/DIS 8708 “Crude Petroleum Oil - Determination of Distillation Characteristics Using 15 Theoretical Plates Columns” by the International Organization for Standardization (8). The ANS521 crude oil can be obtained from the National Environmental Technology Applications Center's (NETAC) Bioremediation Products Evaluation Center (BPEC), University of Pittsburgh Applied Research Center, 615 William Pitt Way, Pittsburgh, PA, 15238, (412) 826-5511. The crude oil is heated to 190 °C (374 °F) under atmospheric pressure. The system is then cooled and placed under vacuum (or under an atmospheric pressure of 20 mm Hg) for the final distillation to an atmospheric equivalent boiling point of 272 °C (521 °F).
4.3.3 Preparation of mineral nutrient solution. If a commercial product is strictly a microbial agent and does not contain its own nutrients, a mineral nutrient solution will be provided if requested by the product manufacturer or vendor. If a commercial product contains its own nutrients, no further nutrients will be added. The nutrient solution is a modified salt solution and is described below.
4.3.3.1 Nutrient preparation:
1. N&P Salts. The following salts are added to distilled water and made up to a 1,000-ml volume. Adjust final pH to 7.8. The solution is sterilized by autoclaving at 121 °C at 15 psig for 20 minutes or by filtering through a sterile 0.22 µm membrane filter.
Na2 HPO4.2H2 - 18.40 g KNO3 - 76.30 g2. MgSO4.7H2 O solution. Dissolve 22.50 g in 1,000 ml distilled water. The solution is sterilized by autoclaving at 121 °C at 15 psig for 20 minutes.
3. CaCl2 solution. Dissolve 27.50 g in 1,000 ml of distilled water. The solution is sterilized by autoclaving at 121 °C at 15 psig for 20 minutes.
4. FeCl3•6H2 O solution. Dissolve 0.25 g in 1,000 ml of distilled water. The solution is sterilized by autoclaving at 121 °C at 15 psig for 20 minutes.
5. Trace Element Solution. The following salts are added to distilled water and made up to a 1,000-ml volume. The solution is sterilized by autoclaving at 121 °C at 15 psig for 20 minutes.
MnSO4.H2 O - 30.2 mg H3 BO3 - 57.2 mg ZnSO4.7H2 O - 42.8 mg (NH4)6Mo7(O2)4 - 34.7 mgThe pH of the nutrient solution is adjusted with a pH meter calibrated at room temperature (approximately 25 °C) using commercial buffers of pH 4.0, 7.0, and 10.0 (Fisher Scientific), as appropriate, prior to use. The pH is adjusted with concentrated HCl or 10 M NaOH, as appropriate.
4.3.3.2 Final concentrations: Ten (10) ml of solution 1 and 2 ml of solutions 2-5 are added to non-sterile seawater and made up to a 1,000-ml volume immediately prior to test start-up. This seawater/mineral nutrient solution is used for all flasks containing products requiring nutrient supplements and for the flasks containing no commercial additive. Seawater without the above nutrient solutions is used for products containing their own source of nutrients.
4.4 Pretest preparation.
4.4.1 Experimental setup.
4.4.1.1 The procedure consists of an experimental shaker flask setup and the specific set of microbiological and chemical analyses that are performed on individual product samples. The following test flasks (labeled with unique identifiers) are prepared and set up on a gyratory shaker at day 0 to reflect the following treatment design:
| Treatment | No. of samples at sampling times | Total No. of analytical determinations | ||||
|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 28 | Microbial counts | Gravimetric | GC/MS | |
| Control | 3 | 3 | 3 | 9 | 9 | 9 |
| Nutrient | 3 | 3 | 3 | 9 | 9 | 9 |
| Product | 3 | 3 | 3 | 9 | 9 | 9 |
Control = Oil + Seawater
Nutrient = Oil + Seawater + Nutrient
Product = Oil + Seawater + Product ( + Nutrient, if required).
4.4.1.2 For each test, a sheet listing the number of flasks, types of controls, number of replicates, product to be tested, and other information is prepared. The following steps should be adhered to for the experimental setup:
1. Borosilicate glass Erlenmeyer flasks (250-ml) are thoroughly cleaned and autoclaved for 20 minutes at 120 °C at 15 psi, then dried in the drying oven.
2. Flasks are labeled with the appropriate code: product or control, sample day, and letter indicating replicate.
3. 100 ml of seawater is added to each flask.
4. For nutrient and product treatments that require the addition of nutrients, seawater containing the nutrient solution is prepared.
5. Pasteur pipettes should be sterilized in advance. Break off the tip to provide a larger opening prior to sterilization.
6. Pour the approximate amount of oil to be used from the large stock bottle into a sterile beaker. Keep the beaker covered when oil is not being removed.
7. The labeled flasks containing seawater and other additions, as necessary, are placed on the balance. The flask is tared. The appropriate amount of oil (0.5 g) is added drop by drop using a sterile Pasteur pipette with the tip broken off to provide a wider opening. Care is taken to avoid splashing the oil or getting it on the sides of flasks. Precautions are taken when handling and charging the flasks to minimize the likelihood of contamination by exogenous microbes. This includes using a new sterile pipette for each series of flasks.
8. The weight of the oil is recorded in the laboratory notebook.
9. The product is prepared and added to the appropriate flasks according to the manufacturer's or vendor's instructions.
10. Flasks are carried upright and carefully placed in the holders on the shaker table to minimize the amount of oil that might adhere to the side of the flasks. Flasks in which a significant amount of oil is splashed on the sides are redone.
11. The prepared flasks are shaken at 200 rpm at 20 °C until such time that they will be removed for sampling.
4.4.2 Sampling. The control and treatments (nutrient and product flasks) are sampled three times over a 28-day period: day 0, day 7, and day 28. The entire flask is sacrificed for analysis; a 0.5-ml aliquot is removed from each flask for the microbiological analysis and the remainder of each flask is used for the chemical analysis. Specific procedures for both the microbiological and chemical analysis are described below. At the time of each sampling event, physical observations of each flask should be recorded.
4.5 Microbiological analysis. To monitor the viability of the microbial cultures being studied, microbial enumerations of hydrocarbon degraders are performed at each sampling event using a microtiter MPN determination. This is used as an indicator of the relative change in biomass. This test design relies on using growth response as an indication of enhanced activity as compared to a “no addition” control.
4.5.1 Media preparation. Media for microbial enumerations are carefully prepared according to manufacturer's or other instructions and sterilized using appropriate methods.
4.5.1.1 General media treatment: Buy Bushnell-Haas (B-H) broth in quantities to last no longer than one year. Use media on a first-in, first-out basis. When practical, buy media in quarter-pound multiples, rather than one-pound multiples to keep supply sealed as long as possible. Keep an inventory of media, including kind, amount, lot number, expiration date, date received, and date opened. Check inventory before reordering media. Discard media that are caked, discolored, or show other deterioration.
4.5.1.2 Sterile saline (pH adjusted):
1. Weigh 30 g of NaCl.
2. Dissolve in enough water to make 1,000 ml.
3. Adjust pH to 8.0 with NaOH (10M and 0.5M).
4. Sterilize by autoclaving for 15 minutes at 15 psig.
4.5.1.3 Standard nutrient concentrate (add 1 ml to each 100 ml of Bushnell-Haas medium for MPNs):
1. Weigh compounds listed below, dissolve in DIH2 O, dilute to 1 liter.
Potassium Phosphate, monobasic KH2 PO4 - 0.633 g Potassium Phosphate, dibasic K2 HPO4 - 1.619 g Sodium Phosphate, dibasic Na2 HPO4 - 2.486 g Ammonium Chloride NH4 Cl - 3.850 g Magnesium Sulfate, heptahydrate MgSO4·7H2 O - 4.500 g Calcium Chloride, dihydrate CaCl2·2H2 O - 7.290 g Ferric Chloride, hexahydrate FeCl3·6H2 O - 0.250 g Trace Elements Manganese Sulfate, monohydrate MnSO2·H2 O - 6.04 mg Boric Acid H3 Bo3 - 11.44 mg Zinc Sulfate, heptahydrate ZnSO4·7H2 O - 8.56 mg Ammonium Moybdate, tetrahydrate (NH4)6Mo7 O24·4H2 O - 6.94 mg2. Adjust pH to 6.0.
3. Stir solution for approximately 3 hours, then filter through a Buchner funnel using #1 paper, which will retain approximately 3.8 g of insolubles.
4. Then filter through a 0.45 micron filter into sterile bottles.
5. Cap bottles, label, and store in refrigerator until used.
4.5.1.4 Quality assurance/Quality control (QA/QC):
1. Periodically check the effectiveness of sterilization using commercially available tapes or Bacillus stearothermophilus spore suspensions, following the instructions with these products.
2. Maintain a media log book that includes the dates, kinds and amounts of media made, pH, and any problems or observations.
3. Before use, check plates and tubes for signs of contamination, drying, or other problems.
4.5.1.5 Safety/Special precautions:
1. Note any safety or other precautions for particular media.
2. Note precautions to be followed when using the autoclave.
3. Use gloves and other protective clothes when handling media.
4. Use care in handling hot media.
4.5.2 Microbial enumeration. Standardized techniques for performing Most Probable Number microbial enumerations are described below.
4.5.2.1 Dilutions:
1. Prior to sacrificing each flask, remove 0.5 ml of water from each flask and add it to a tube of 4.5 ml sterile phosphate buffer (1:10 dilution) as prepared in the Standard Methods for the Examination of Water and Wastewater (9). Using sterile technique, mix and perform serial dilutions (0.5 ml of previous dilution to 4.5 ml of sterile phosphate buffer) to 10−9 dilution.
4.5.2.2 Inoculating MPN plates (oil degrader):1. Prepare sufficient sterile 0.4 M NaCl (23.4 g NaCl/1,000 ml B-H) and B-H at pH 7.0 to fill the number of wells required for the test (1.75 ml/well).
2. Using sterile technique, add 1.75 ml of B-H broth to each well.
3. Label the top of the plate with the proper dilution for each row.
4. Add 0.1 ml of fluid from each dilution tube to each well in the appropriate row, starting with the most dilute.
5. After adding the fluid to all the wells, add 20 µl of sterilized No. 2 fuel oil to the top of each well.
6. Incubate each plate at 20 °C.
7. After 14 days of incubation, add 100 µl of p-iodotetrazolium violet dye (50 mg/10 ml of D.I. water) to each well to determine growth.
8. View plates against a white background to determine if color is present. Development of a purple or pink color upon standing for 45 minutes constitutes a positive test.
9. Record the number of positive wells and the dilutions at which they occur.
10. Enter data into a computerized enumeration method using “MPN Calculator” software program (version 2.3 or higher) by Albert J. Klee, U.S. EPA Office of Research and Development, Risk Reduction Engineering Laboratory, Cincinnati, OH.
4.5.2.3 Quality assurance/Quality control:
1. Check pH of medium before preparing wells (pH should be approximately 8.0). Adjust pH, if necessary, with dilute NaOH.
2. Keep prepared tetrazolium violet dye solution in the refrigerator in an amber bottle when not in use.
3. Have all laboratory personnel periodically run MPNs on the same sample to test precision.
4.5.2.4 Safety/Special precautions:
1. Use sterile technique in preparing solutions, dilutions, plates, and MPN wells.
2. Do not pipette potentially hazardous solutions by mouth.
3. Autoclave all plates and wells before discarding.
4.6 Chemical analysis of oil composition.
4.6.1 Sample procedure. After 0, 7, and 28 days of incubation on a rotary shaker, the appropriate flasks are sacrificed and extracted with dichloromethane and spiked with a surrogate recovery standard. A 10-ml aliquot of the DCM layer is used for the gravimetric analysis. If significant biodegradation is evident in the results of the gravimetric analysis, then a solvent exchange into hexane takes place prior to the GC/MS analysis. Follow steps 1-19 below when preparing for the chemical analysis.
1. After 0, 7, and 28 days of rotary shaking and incubating at 20 °C, the reaction vessels are sacrificed. Prior to the chemical analysis, a 0.5-ml sample of the aqueous phase is removed for the microbiological analysis (see Microbial Enumeration above).
2. A surrogate recovery standard is prepared in the following manner: 1,000 mg of d10-phenanthrene and 1,000 mg of 5α-androstane are measured into a 500-ml volumetric flask and DCM is added to the mark to produce a 2,000-ng/µl stock solution.
3. A 100-µl aliquot of the surrogate solution is added to each test flask. The final concentration of surrogates in each flask is approximately 4 ng/µl of solvent in the final extract. The aliphatics and marker data should be corrected for percent recovery of the 5α-androstane surrogate and the aromatics for the d10-phenanthrene surrogate.
4. The contents of the flask are placed into a 250-ml separatory funnel.
5. Measure a total volume of 50 ml DCM for use in the extraction. Use 3 10-ml fractions to rinse the flask into the funnel and transfer the remaining aliquot of DCM to the funnel.
6. Stopper and mix vigorously by shaking (approximately 50 times) while ventilating properly.
7. Each funnel is set aside to allow the DCM and water layers to partition. This may take 5-10 minutes for some products, or up to 3 hours if the product has caused the formation of an emulsion.
8. Drain the first 10 ml of the DCM (bottom) layer, collect, cap, uniquely label, and use for gravimetric analysis (see below). Drain the remaining 40 ml and dry it by passing it through a funnel packed with anhydrous sodium sulfate.
9. Assemble a Kuderna-Danish (KD) concentrator by attaching a Snyder column to an evaporation flask with a graduated concentrator tube. Align vertically and partially immerse concentrator tube in a water bath (10). Set the water bath to the appropriate temperature to maintain proper distillation.
10. Collect the de-watered extract into the KD concentrator.
11. Evaporate DCM to approximately 10 ml, then add approximately 50 ml of the exchange solvent (hexane) and concentrate the volume to 10 ml.
12. Rinse the flask into the concentrator tube with 50 ml hexane and concentrate to 10 ml. Repeat one more time with 50 ml of hexane.
13. Remove concentrator tube with the recovered 10 ml of sample volume. The heavier residual material should be present as a precipitate (bottom layer).
14. Centrifuge to aid the separation of the hexane from the precipitant fraction.
15. Place hexane-soluble fraction (top layer) - approximately 1.0 ml - into a GC/MS vial for analysis (see GC/MS Analysis Procedure below). If column fouling and deterioration of separation characteristics occur, an alumina column sample cleanup method can be considered (see Alternative GC/MS Sample Cleanup Procedure below).
16. Analyze by GC/MS using the conditions determined by the U.S. EPA Risk Reduction Engineering Laboratory, Water and Hazardous Waste Treatment Research Division, in Cincinnati, OH, which follows U.S. EPA Method 8270 (see GC/MS Analysis Procedure below).
17. Calculate surrogate recovery. If surrogate recovery is less than 85 percent for the marker relative to the surrogate recovery standard (d10-phenanthrene), then the water layer should be extracted again using three separate extractions with DCM. Pool the three extractions with original extract and concentrate to 10 ml, and reanalyze by GC/MS.
18. Drain the seawater into a storage sample vial/container.
19. Seal the vial with a Teflon-lined cap and store frozen. This water layer is kept in case additional extractions are necessary.
4.6.2 Gravimetric analysis. The initial means to evaluate the effectiveness of a bioremediation agent for oil spill response is through gravimetric analysis. A statistically significant difference (p <0.05) in analytical weight of the oil from the control system as compared to the analytical weight of the oil treated with a bioremediation agent indicates biodegradation has successfully occurred. Hence, the disappearance of oil should be accompanied by significant decreases in total oil residue weight of extractable materials versus a control. If no significant decrease in oil residue weight is observed, the need to perform further chemical analysis should be evaluated. Follow steps 1-3 to conduct the gravimetric analysis.
1. The 10 ml of DCM extract (from Sample Procedure step 8 above) is placed in a small vial and concentrated to dryness by nitrogen blowdown techniques using a steady stream of nitrogen (pre-purified gas). If the oil is severely biodegraded, a larger volume of DCM (>10 ml) may be necessary for the gravimetric analysis.
2. The residue is weighed 3 times for the gravimetric weight of oil. Record the weight of the oil.
3. Compare statistically (p <0.05) the weight of the product treatment versus the weight of the control from each respective time period. If a significant decrease is observed in the sampling (flask containing bioremediation agent) weight, then proceed with the remainder of the sample procedure.
4.6.3 GC/MS analysis. Often, analysis of saturated and aromatic hydrocarbons by capillary gas chromatography of DCM extracts leads to column fouling and deterioration of separation characteristics. An alternative, simple “one-step” alumina sample cleanup procedure can be performed on oil before injection; this cleanup removes both asphaltenes and polar compounds and can be applied to DCM extracts as well. This procedure is described in steps 1-11 below.
4.6.3.1 Alternative GC/MS sample cleanup procedure:
1. Weigh 4.0 g alumina (neutral, 80-200 mesh) into scintillation vials covered loosely with aluminum foil caps. Prepare one scintillation vial per sample. Heat for 18 hours at 300 °C or longer. Place in a desiccator of silica until needed.
2. Add 5.0 ml of DCM to a glass luerlok multi-fit syringe (e.g., BD #2471) with stopcock (e.g., Perfectum #6021) in closed position, stainless steel syringe needle (18 gauge), and PTFE frits. Clamp in a vertical position.
3. Transfer 4.0 g of prepared alumina to a plastic weighing boat and fill syringe slowly while applying continuous vibration (e.g., Conair # HM 11FF1).
4. Add a second PTFE frit and push into place on top of the alumina bed.
5. Drain 5.0 ml DCM to the top level of the column frit to await sample addition and discard DCM.
6. Weigh 50 mg ±0.1 mg ANS521 oil into a tared vial.
7. Premeasure 10 ml of DCM into a graduated cylinder. Add 0.2 to 0.3 ml of the DCM to the tared oil vial. Mix and transfer solvent to the column bed with a Pasteur pipette. Open stopcock and collect in a 10-ml volumetric flask. Repeat until approximately 1.0 ml (do not exceed 1.0 ml) of DCM has rinsed the vial and inner walls of the syringe body into the 10-ml flask.
8. Transfer balance of DCM from the graduated cylinder to the column and regulate the solvent flow rate to approximately 1 to 2 ml/minute. Collect all eluent in the 10-ml flask.
9. Transfer a known volume of eluent to another scintillation vial and blow down to dryness (nitrogen).
10. Determine and record weight.
11. Dissolve in 1.0 ml hexane for the GC/MS analysis procedure (see below).
4.6.3.2 GC/MS analysis procedure:
Immediately prior to injection, an internal standard solution of four deuterated compounds is spiked into the sample extracts and injected. Samples are quantified using the internal standard technique (10) for both the aliphatic and aromatic fractions of the oil extracts in order to provide sufficient information that the oil is being degraded. To help ensure that the observed decline in target analytes is caused by biodegradation rather than by physical loss from mishandling or inefficient extraction, it is necessary to normalize the concentrations of the target analytes via a “conserved internal marker.” Conserved internal markers that have been found useful for quantification are C2- or C3-phenanthrene, C2-chrysene, and C3017α(H),21β(H)-hopane. Deuterated internal standards are used to calculate the relative response factor (RRF) for the target analyte(s). To compute the “normalized concentrations,” the target analyte concentration at a given sampling time is simply divided by the selected conserved analyte concentration at the same sampling time (11). Conduct the GC/MS analysis using the following procedure.
1. One (1) ml of the hexane extract (from Sample Procedure step 15 above) is placed into a 1.5-ml vial for use on the autosampler of the GC/MS instrument.
2. To this solution, 20 µl of a 500-ng/µl solution of the internal standards is added and the vial is capped for injection. The final concentration of the internal standards in each sample is 10 ng/µl. This solution contains 4 deuterated compounds: d8-naphthalene, d10-anthracene, d12-chrysene, and d12-perylene.
3. At the start of any analysis period, the mass spectrometer (MS) is tuned to PFTBA by an autotune program, such as the Hewlett-Packard quicktune routine, to reduce operator variability. Set the GC/MS in the SIM mode at a scan rate of 1.5 scans/second to maximize the linear quantitative range and precision of the instrument. Set all other conditions to those specified in Instrument Configuration and Calibration section below.
4. An instrument blank and a daily standard are analyzed prior to analysis of unknowns. Internal standards are combined with the sample extracts and coinjected with each analysis to monitor the instrument's performance during each run.
5. Information that should be included on the acquisition form include operator's name and signature, date of extraction, date and time of autotune, date of injection(s), instrument blank, daily standard mix injection, GC column number, and standards for the 5-point calibration curve.
6. If the instrument is operated for a period of time greater than 12 hours, the tune will be checked and another daily standard analyzed prior to continuing with analyses.
Table 6 - Analytes Listed Under the Corresponding Internal Standard Used for Calculating RRFs
| Internal Standard | d8-naphthalene | d10-anthracene | d12-chrysene | d12-perylene |
|---|---|---|---|---|
| Alkanes | nC10-nC15 | nC16-nC23 | nC24-nC29 | nC30-nC35. |
| Pristane | C3017β(H), 21α(H)-hopane. | |||
| Phytane | ||||
| 5α-androstane | ||||
| Aromatics | Naphthalene | Dibenzothiophene | Fluoranthene | Benzo(b)fluoranthene. |
| Fluorene | Pyrene | Benzo(k)fluoranthene. | ||
| Anthracene | Chrysene | Benzo(e)pyrene. | ||
| Phenanthrene | Benzo(a)pyrene. | |||
| Perylene. | ||||
| Indeno(g,h,i)pyrene. | ||||
| Dibenzo(a,h) anthracene. | ||||
| Benzo(1,2,3-cd)perylene. |
7. The MS is calibrated using a modified version of EPA Method 8270 (10). Specifically, the concentrations of internal standards are 10 ng/µl instead of 40 ng/µl. A five-point calibration curve is obtained for each compound listed in table 6 prior to sample analysis at 1, 5, 10, 25, and 50 ng/µl. A 5-point calibration must be conducted on a standard mix of compounds to determine RRFs for the analytes. The standard mix (excluding the marker) for this calibration curve may be obtained from Absolute Standards, Inc., 498 Russell St., New Haven, CT, 06513, (800) 368-1131. If C3017β(H),21α(H)-hopane is used, it may be obtained from Dr. Charles Kennicutt II, Geochemical and Environmental Research Group, Texas A&M University, 833 Graham Rd., College Station, TX, 77845, (409) 690-0095.
8. Calculate each compound's relative response factor to its corresponding deuterated internal standard indicated above, using the following equation:
RRF = (Ax Cis)/(Ais Cx)(6) where: RRF = relative response factor Ax = peak area of the characteristic ion for the compound being measured (analyte) Ais = peak area of the characteristic ion for the specific internal standard Cx = concentration of the compound being measured (ng/µl) Cis = concentration of the specific internal standard (10 ng/µl). (This concentration is a constant in this equation for the calibration curve.)9. Identify each analyte based on the integrated abundance from the primary characteristic ion indicated in table 7.
10. Quantitate each analyte using the internal standard technique. The internal standard used shall be the one nearest the retention time of that of a given analyte (Table 8).
Table 7 - Primary Ions Monitored for Each Target Analyte During GC/MS Analysis
| Compound | Ion |
|---|---|
| n-alkanes (C10-C35) | 85 |
| Pristane | 85 |
| Phytane | 85 |
| Naphthalene | 128 |
| C1-naphthalenes | 142 |
| C2-naphthalenes | 156 |
| C3-naphthalenes | 170 |
| C4-naphthalenes | 184 |
| Fluorene | 166 |
| C1-fluorenes | 180 |
| C2-fluorenes | 194 |
| C3-fluorenes | 208 |
| Dibenzothiophenes | 184 |
| C1-dibenzothiophenes | 198 |
| C2-dibenzothiophenes | 212 |
| C3-dibenzothiophenes | 226 |
| Anthracene | 178 |
| Phenanthrene | 178 |
| C1-phenanthrenes | 192 |
| C2-phenanthrenes | 206 |
| C3-phenanthrenes | 220 |
| Fluoranthene/pyrene | 202 |
| C1-pyrenes | 216 |
| C2-pyrenes | 230 |
| Chrysene | 228 |
| C1-chrysenes | 242 |
| C2-chrysenes | 256 |
| Hopanes (177 family) | 177 |
| Hopanes (191 family) | 191 |
| Steranes (217 family) | 217 |
| Benzo(b)fluoranthene | 252 |
| Benzo(k)fluoranthene | 252 |
| Benzo(e)pyrene | 252 |
| Benzo(a)pyrene | 252 |
| Perylene | 252 |
| Ideno(g,h,i)pyrene | 276 |
| Dibenzo(a,h)anthracene | 278 |
| Benzo(1,2,3-cd)perylene | 276 |
| d8-naphthalene | 136 |
| d10-anthracene | 188 |
| d10-phenanthrene | 188 |
| d12-chrysene | 240 |
| d12-perylene | 264 |
| α-androstane | 260 |
Table 8 - Analytes and Reference Compounds
| Compound | Reference compound | Compound | Reference compound |
|---|---|---|---|
| n-C10 | n-C10 | C2-naphthalene | Naphthalene. |
| n-C11 | n-C11 | C3-naphthalene | Naphthalene. |
| n-C12 | n-C12 | C4-naphthalene | Naphthalene. |
| n-C13 | n-C13 | Fluorene | Fluorene. |
| n-C14 | n-C14 | C1-fluorene | Fluorene. |
| n-C15 | n-C15 | C2-fluorene | Fluorene. |
| n-C16 | n-C16 | C3-fluorene | Fluorene. |
| n-C17 | n-C17 | Dibenzothiophene | Dibenzothiophene. |
| Pristane | Pristane | C1-dibenzothiophene | Dibenzothiophene. |
| n-C18 | n-C18 | C2-dibenzothiophene | Dibenzothiophene. |
| Phytane | Phytane | C3-dibenzothiophene | Dibenzothiophene. |
| n-C19 | n-C19 | Phenanthrene | Phenanthrene. |
| n-C20 | n-C20 | Anthracene | Anthracene. |
| n-C21 | n-C21 | C1-phenanthrene | Phenanthrene. |
| n-C22 | n-C22 | C2-phenanthrene | Phenanthrene. |
| n-C23 | n-C23 | C3-phenanthrene | Phenanthrene. |
| n-C24 | n-C24 | Fluoranthene | Fluoranthene. |
| n-C25 | n-C25 | Pyrene | Pyrene. |
| n-C26 | n-C26 | C1-pyrene | Pyrene. |
| n-C27 | n-C27 | C2-pyrene | Pyrene. |
| n-C28 | n-C28 | Chrysene | Chrysene. |
| n-C29 | n-C29 | C1-chrysene | Chrysene. |
| n-C30 | n-C30 | C2-chrysene | Chrysene. |
| n-C31 | n-C31 | Benzo(b)fluoranthene | Benzo(b)fluoranthene. |
| n-C32 | n-C32 | Benzo(k)fluoranthene | Benzo(k)fluoranthene. |
| n-C33 | n-C33 | Benzo(e)pyrene | Benzo(e)pyrene. |
| n-C34 | n-C34 | Benzo(a)pyrene | Benzo(a)pyrene. |
| n-C35 C3017α,21β-hopane | n-C35 C3017α,21β-hopane | Perylene ideno(g,h,i)pyrene | Perylene ideno(g,h,i)pyrene. |
| 5α-androstane | 5α-androstane | Dibenzo(a,h)anthracene | Dibenzo(a,h)anthracene. |
| C1-naphthalene | Naphthalene | Benzo(1,2,3-cd)perylene | Benzo(1,2,3-cd)perylene. |
11. Use equation 7 to calculate the concentration of analytes in ng/mg (ppm) oil:
Concentration (ng/mg) = (Ax Is Vt × 1,000)/(Ais(RRF)Vi Mo)(7) where: Ax = peak area of characteristic ion for compound being measured Is = amount of internal standard injected, in ng (i.e., 20 ng) Vt = volume of the total DCM extract (50 ml) Ais = peak area of the characteristic ion of the internal standard RRF = relative response factor Vi = volume of the extract injected (2 µl) Mo = total mass of the oil added to the flask, mg12. Compute the “normalized concentrations” for each target analyte concentration at a given sampling time (equation 7) by simply dividing by the conserved internal marker concentration at the same sampling time.
4.6.4 Generally accepted laboratory procedures. Samples are immediately logged into the laboratory, where they will be given a unique sample identification based on Julian data and the number logged in. Prior to the analysis of any experimental samples, a five-point standard curve is prepared. One of the mid-range standard curve concentration levels is analyzed daily before sample analysis as a continuing standard. RRFs for all target analytes should be within 25% of the standard curve response values at day 0, and at any sampling event the check standard percent difference from the initial five-point calibration must not exceed 20% between the before and after daily standard mix (see below). The collected GC/MS data are initially processed by a macro routine, which performs extracted chromatographic plots of the target compounds, integrates the target compounds, and shows integration results to include tabular numbers. The integration values are then transferred to a spreadsheet format to be quantified. Because of the complexity of the analyte matrix (oil), a very high degree of manual verification and reintegration of the spectral data is required.
4.6.5 QA/QC procedures. The reliability of this method is dependent on the QA/QC procedures followed. Before and after each analytical batch (approximately 10 samples), analyze one procedural blank, one duplicate, and one calibration verification standard (10 ng/µl). Analyze one reference crude oil standard. The instrument's performance and reproducibility are validated routinely in this manner. Surrogate recoveries should be within 70 to 120%, and duplicate relative percent difference values should be ±20%. A control chart of the standard oil should be prepared and monitored. Variations of analytes in the control chart should be no more than 25% from the historical averages. Injection port discrimination for n-C25 and greater alkanes must be carefully monitored; the ratio of RRF n-C32/RRF n-C21 alkanes should not be allowed to fall below 80%. The mass discrimination can be reduced by replacing the quartz liner in the injection port after every analytical batch. The instrument's performance and reproducibility are validated routinely by analyzing the reference crude oil standard. All analyses are recorded in instrument logs detailing operating conditions, date and time, file name, etc. After analysis, the sample extracts are archived at refrigeration temperatures. To document QA/QC, the following information is contained in the detailed quantitative reports: average RRF derived from the standard curve; RRF from the daily standard; percent relative standard deviation; area of target analyte; concentration determined both on a weight and volume basis; and values for any surrogates and internal standards.
4.6.6 Instrument configuration and calibration. A 2-ml aliquot of the hexane extract prepared by the above procedure is injected into a GC/MS instrument, such as the Hewlett-Packard 5890/5971 GC/MS (recommended for use). This instrument should be equipped with a DB-5 capillary column (30 m, 0.25-mm I.D., and 0.25-µm film thickness) and a split/splitless injection port operating in the splitless mode. Table 9 summarizes the temperature program used for the analysis. This temperature program has been optimized to give the best separation and sensitivity for analysis of the desired compounds on the instrument. Prior to the sample analysis, a five-point calibration must be conducted on a standard mix of the compounds listed in table 7 to determine RRFs for the analyses.
Table 9 - Operating Conditions and Temperature Program of GC/MS
| Operating conditions |
|---|
| Injector port - 290 °C |
| Transfer line - 320 °C |
| Total run time - 73 minutes |
| Column flow rate (He) - 1.0 ml/minute |
| Temperature Program | |||||
|---|---|---|---|---|---|
| Level | Temp. 1, °C | Time 1, minutes | Rate, °C/minute | Temp 2, °C | Time 2, minutes |
| Level 1 | 55 | 3 | 5 | 280 | 5 |
| Level 2 | 280 | 0 | 3 | 310 | 10 |
4.7 Statistical analysis. The determination of a bioremediation agent's effectiveness will be partially based upon the results of a statistical analysis of the shaker flask experiment. The experimental design for this test is a two factorial design. This two-way analysis of variance (ANOVA) will be used to determine data trends. The statistical method is designed to test various types of bioremediation treatments including microbial, nutrient, enzyme, and combination products. The following is a summary of the statistical methods to be used to evaluate the analytical data obtained from all product tests. The experimental design, data analysis methodology, interpretation of results, required documentation, and a numeric example are outlined below.
4.7.1 Experimental design. The experimental design for this test is known as a factorial experiment with two factors. The first factor is product/control group; the second factor is time (measured in days). For example, if two groups (product A and a non-nutrient control) are tested at each of three points in time (day 0, 7, and 28), the experiment is called a 2 × 3 factorial experiment. There will be three replications (replicated shaker flasks) of each group-time combination.
4.7.2 Data analysis methods. For each analyte and each product used, a product is considered a success by the demonstration of a statistically significant difference between the mean analyte degradation by the product and the mean analyte degradation by the non-nutrient control. Such a determination will be made by performing an ANOVA on the sample data. The technical aspects of this procedure are outlined in Snedecor and Cochran (12). Most statistical software packages support the use of two-way ANOVA. However, the format required for the input data differs among the various commercial packages. Whichever package is used, the following ANOVA table will be provided as part of the output. In the Degree of Freedom column of table 10, p = the number of product/control groups, t = the number of days at which each group is analyzed, and n = the number of replications. For the example of the 2 × 3 factorial experiment discussed above, p = 2, t = 3, and n = 3. The significance of the F-statistics (as indicated by their corresponding p-values) are used to interpret the analysis.
Table 10 - Two-Way ANOVA Table
| Source | Degree of freedom (df) | Sum of squares | Mean square | F-Statistic | p-Value |
|---|---|---|---|---|---|
| Group | p-1 | SSG | MSG-MSG/MSE | MSG/MSE | 1 |
| Time | t-1 | SST | MST-MST/MSE | MST/MSE | 1 |
| Interaction | (p-1)(t-1) | SSI | MSI-MSI/MSE | MSI/MSE | 1 |
| Error | pt(n-1) | SSE | MSE-SSE | ||
| Total | npt-1 | SSTOT |
1 To be determined from the value of the F-statistic.
4.7.3 Interpretation. 4.7.3.1 If the F-statistic for the interaction is significant at the 0.05 level (i.e., p-value is less than 0.05), the data indicate that the mean response of at least two groups being tested differ for at least one point in time. In order to find out which groups and at which points in time the difference occurs, pairwise comparisons between the group means should be conducted for all time points. These comparisons can be made using protected least squared difference (LSD) or Dunnett mean separation techniques. The protected LSD procedure is detailed in Snedecor and Cochran (12); the Dunnett procedure is outlined in Montgomery (13). For both methods, the mean square error (MSE) from the two-way ANOVA table should be used to compute the separation values.
4.7.3.2 If the F-statistic for the interaction is not significant at the 0.05 level (i.e., p-value not less than 0.05), but the F-statistic for the group is significant (i.e., p-value is less than 0.05), the data indicate that any differences that exist among the group means are consistent across time. To find out which group means differ, a pairwise comparison of the group means should be carried out by pooling data across all points in time. Again, the MSE from the two-way ANOVA table should be used to compute the separation values.
4.7.3.3 If the F-statistic corresponding to both interaction and group are not significant at the 0.05 level, the data indicate no difference between the group means at any point in time. In this case, no further analysis is necessary.
4.7.3.4 Finally, Snedecor and Cochran (12) use caution concerning the use of multiple comparisons. If many such comparisons are being conducted, then about 5% of the tested differences will erroneously be concluded as significant. The researcher must guard against such differences causing undue attention.
4.7.4 Required documentation. 4.7.4.1 The following documents should be included to summarize the findings from a product test.
1. Data listings for each analyte that was analyzed. These should show all raw data.
2. A table of summary statistics for each analyte. The table should include the mean, standard deviation, and sample size for each group at each day.
3. An ANOVA table for each analyte. The table should be of the same format as table 10.
4. A clear summary of the mean separations (if mean separations were necessary). The mean separation methods (LSD or Dunnett), the significance level, the minimum significant difference value, and the significant differences should be clearly marked on each output page.
5. All computer outputs should be included. No programming alterations are necessary. The specific computer package used to analyze the data should be included in the report.
Example.An analysis of the total aromatic data (in ppm) was conducted for the following three groups:Group 1: Non-nutrient Control
Group 2: Nutrient Control
Group 3: Test Product
4.7.4.2 The raw data are shown in table 11. Note the three replications for each group-time combination.
Table 11 - Product Test Data, Total Aromatics (ppm)
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Day 0 | 8153 | 7912 | 7711 |
| 8299 | 8309 | 8311 | |
| 8088 | 8111 | 8200 | |
| Day 7 | 8100 | 7950 | 6900 |
| 8078 | 8200 | 6702 | |
| 7999 | 8019 | 5987 | |
| Day 28 | 8259 | 8102 | 4000 |
| 8111 | 7754 | 3875 | |
| 8344 | 7659 | 3100 |
4.7.4.3 Table 12 gives the summary statistics (number of observations, means, and standard deviations) for each group-time combination.
Table 12 - Summary Statistics for Product Test Data Total Aromatics (ppm)
| Time | Product | n | Mean | Standard deviation |
|---|---|---|---|---|
| Day 0 | Group 1 | 3 | 8,180.0 | 108.1 |
| Group 2 | 3 | 8,110.7 | 198.5 | |
| Group 3 | 3 | 8,074.0 | 319.2 | |
| Day 7 | Group 1 | 3 | 8,059.0 | 53.1 |
| Group 2 | 3 | 8,056.3 | 129.1 | |
| Group 3 | 3 | 6,529.7 | 480.3 | |
| Day 28 | Group 1 | 3 | 8,238.0 | 117.9 |
| Group 2 | 3 | 7,838.3 | 233.2 | |
| Group 3 | 3 | 3,658.3 | 487.6 |
4.7.4.4 Table 13 shows the results of the two-way ANOVA.
Table 13 - Example Two-Way ANOVA Table
| Source | df | Sum of squares | Mean square | F-statistic | p-value |
|---|---|---|---|---|---|
| Group | 2 | 23,944,856.41 | 11,972,428.70 | 151.94 | 0.0001 |
| Time | 2 | 10,954,731.19 | 5,477,365.59 | 69.51 | 0.0001 |
| Interaction | 4 | 19,347,589.04 | 4,836,897.26 | 61.39 | 0.0001 |
| Error | 18 | 1,418,303.33 | 78,794.63 | ||
| Total | 26 | 55,665,480.96 |
4.7.4.5 From table 13, it can be seen that the F-statistic for interaction is significant (F = 61.39, p = 0.0001). This indicates that group differences exist for one or more days. Protected LSD mean separations were then conducted for each day to determine which group differences exist. The results are summarized in table 14. Note that means with the same letter (T grouping) are not significantly different.
Table 14 - Pairwise Protected LSD Mean Separation
| T grouping | Mean | n | Interaction |
|---|---|---|---|
| A | 8,338.0 | 3 | Group 1, Day 28. |
| A | 8,180.0 | 3 | Group 1, Day 0. |
| A | 8,110.7 | 3 | Group 2, Day 0. |
| A | 8,074.0 | 3 | Group 3, Day 0. |
| A | 8,059.0 | 3 | Group 1, Day 7. |
| A | 8,056.3 | 3 | Group 2, Day 7. |
| A | 7,838.3 | 3 | Group 2, Day 28. |
| B | 6,529.7 | 3 | Group 3, Day 7. |
| C | 3,658.3 | 3 | Group 3, Day 28. |
Significant Level = 0.05.
Degrees of Freedom = 18.
Mean Square Error = 78794.63.
Critical Value = 2.10.
Least Significant Difference = 481.52.
4.7.4.6 The grouping letters indicate that the product mean values (group 3) at day 7 and day 28 are significantly different from those of both the nutrient control (group 2) and the non-nutrient control (group 1) for those days. No other significant differences are shown. Therefore, in terms of total aromatic degradation, the test indicates the desired statistically significant difference between the mean of the product and the mean of the non-nutrient control.
5.0 Bioremediation agent toxicity test [Reserved] 6.0 Summary technical product test data format.The purpose of this format is to summarize in a standard and convenient presentation the technical product test data required by the U.S. Environmental Protection Agency before a product may be added to EPA's NCP Product Schedule, which may be used in carrying out the National Oil and Hazardous Substances Pollution Contingency Plan. This format, however, is not to preclude the submission of all the laboratory data used to develop the data summarized in this format. Sufficient data should be presented on both the effectiveness and toxicity tests to enable EPA to evaluate the adequacy of the summarized data. A summary of the technical product test data should be submitted in the following format. The numbered headings should be used in all submissions. The subheadings indicate the kinds of information to be supplied. The listed subheadings, however, are not exhaustive; additional relevant information should be reported where necessary. As noted, some subheadings may apply only to particular types of agents.
I. Name, Brand, or Trademark
II. Name, Address, and Telephone Number of Manufacturer
III. Name, Address, and Telephone Numbers of Primary Distributors
IV. Special Handling and Worker Precautions for Storage and Field Application
1. Flammability.
2. Ventilation.
3. Skin and eye contact; protective clothing; treatment in case of contact.
4. Maximum and minimum storage temperatures; optimum storage temperature range; temperatures of phase separations and chemical changes.
V. Shelf Life
VI. Recommended Application Procedure
1. Application method.
2. Concentration, application rate (e.g., gallons of dispersant per ton of oil).
3. Conditions for use: water salinity, water temperature, types and ages of pollutants.
VII. Toxicity (Dispersants, Surface Washing Agents, Surface Collecting Agents, and Miscellaneous Oil Spill Control Agents)
| Materials Tested | Species | LC50 (ppm) |
|---|---|---|
| Product | Menidia beryllina Mysidopsis bahia 2 |
96-hr. 48-hr. |
| No. 2 fuel oil | Menidia beryllina Mysidopsis bahia |
96-hr. 48-hr. |
| Product and No. 2 fuel oil (1:10) | Menidia beryllina Mysidopsis bahia |
96-hr. 48-hr. |
VIII.(a). Effectiveness (bioremediation agents). Raw data must be reported according to the format shown below. The first column lists the names of the analytes measured by GC/MS (SIM), the surrogate standards, and various ratios and sums. In the next three columns, the concentration of the analytes (ng/mg oil), the concentration of the analytes corrected for the recovery of the surrogate standard (α-androstane for alkanes, d10-phenanthrene for aromatics), and the concentration of corrected analytes normalized against the conserved internal marker, respectively, are reported for the first replicate from the first sampling event. These three columns are each repeated for the next two replicates, giving 9 total columns for the product of interest. The next 9 columns are the same as the product columns except they are for the non-nutrient control. The last nine columns are for the nutrient control. Thus, a total of 28 columns are needed in the spreadsheet. This spreadsheet is for the first sampling event (day 0). Two more identical spreadsheets will be needed for each of the next two sampling events (days 7 and 28). For the statistical analysis, a report showing the two-way analysis of variance (ANOVA) table created by the software used by the investigator must be shown in its entirety along with the name of the software package used. Another printout showing the mean separation table (protected LSD test results) generated by the software must be reported. The statistical analyses are conducted using the sum of the alkane concentrations and the sum of the aromatics concentrations from the raw data table. Thus, two ANOVAs are run for each sampling event, one for total alkanes and one for total aromatics, giving a total of 6 ANOVAs for a product test (2 ANOVAs × 3 sampling events). Only if significant differences are detected by a given ANOVA will it be necessary to run a protected LSD test.
Bioremediation Agent Effectiveness Test Raw Data
[Date: . Testing Date: 0, 7, 28 (Circle One). Initial Oil Weight: .]
| Product Replicate 1 | Product Replicate 2 | |||
|---|---|---|---|---|
| Concentration ng/mg |
Surrogate corrected ng/mg | Normalized to marker ng/mg | ||
| Alkane Analyte | ||||
| n-C10 | ||||
| n-C11 | ||||
| n-C12 | ||||
| n-C13 | ||||
| n-C14 | ||||
| n-C15 | ||||
| n-C16 | ||||
| n-C17 | ||||
| pristane | ||||
| n-C18 | ||||
| phytane | ||||
| n-C19 | ||||
| n-C20 | ||||
| n-C21 | ||||
| n-C22 | ||||
| n-C23 | ||||
| n-C24 | ||||
| n-C25 | ||||
| n-C26 | ||||
| n-C27 | ||||
| n-C28 | ||||
| n-C29 | ||||
| n-C30 | ||||
| n-C31 | ||||
| n-C32 | ||||
| n-C33 | ||||
| n-C34 | ||||
| n-C35 | ||||
| n-C36 | ||||
| α-androstane | ||||
| Total alkanes | ||||
| n-C17:pristane | ||||
| n-C18:phytane | ||||
| Aromatic Analyte: | ||||
| naphthalene | ||||
| C1-naphthalenes | ||||
| C2-naphthalenes | ||||
| C3-naphthalenes | ||||
| C4-naphthalenes | ||||
| dibenzothiophene | ||||
| fluorene | ||||
| C1-fluorenes | ||||
| C2-fluorenes | ||||
| C3-fluorenes | ||||
| C1-dibenzothiophenes | ||||
| C2-dibenzothiophenes | ||||
| C3-dibenzothiophenes | ||||
| phenanthrene | ||||
| anthracene | ||||
| C1-phenanthrenes | ||||
| C2-phenanthrenes | ||||
| C3-phenanthrenes | ||||
| naphthobenzothio | ||||
| C1-naphthobenzothio | ||||
| C2-naphthobenzothio | ||||
| C3-naphthobenzothio | ||||
| fluoranthene | ||||
| pyrene | ||||
| C1-pyrenes | ||||
| C1-pyrenes | ||||
| chrysene | ||||
| benzo(a)anthracene | ||||
| C1-chrysenes | ||||
| c2-chrysenes | ||||
| benzo(b)fluoranth | ||||
| benzo(k)fluoranth | ||||
| benzo(e)pyrene | ||||
| benzo(a)pyrene | ||||
| perylene | ||||
| indeno(1,2,3-cd)per | ||||
| benzo(g,h,i)pyrene | ||||
| dibenz(ah)anthrac | ||||
| α,β-hopane | ||||
| d8-naphthalene | ||||
| d10-phenanthrene | ||||
| d12-chrysene | ||||
| d12-perylene | ||||
| Total aromatics | ||||
| Grav. weight oil | ||||
| No. oil degraders/ml | ||||
VIII.(b). Toxicity (Bioremediation Agents) [Reserved]
IX. Microbiological Analysis (Bioremediation Agents)
X. Physical Properties of Dispersant/Surface Washing Agent/Surface Collecting Agent/Miscellaneous Oil Spill Control Agent:
1. Flash Point: (°F)
2. Pour Point: (°F)
3. Viscosity: ___ at ___ °F (furol seconds)
4. Specific Gravity: ___ at ___ °F
5. pH: (10% solution if hydrocarbon based)
6. Surface Active Agents (Dispersants and Surface Washing Agents) 2
2 If the submitter claims that the information presented under this subheading is confidential, this information should be submitted on a separate sheet of paper clearly labeled according to the subheading and entitled “Confidential Information.”
7. Solvents (Dispersants and Surface Washing Agents)
8. Additives (Dispersants and Surface Washing Agents)
9. Solubility (Surface Collecting Agents)
XI. Analysis for Heavy Metals, Chlorinated Hydrocarbons, and Cyanide (Dispersants, Surface Washing Agents, Surface Collecting Agents, and Miscellaneous Oil Spill Control Agents):
| Compounds | Concentration (ppm) |
|---|---|
| Arsenic | |
| Cadmium | |
| Chromium | |
| Copper | |
| Lead | |
| Mercury | |
| Nickel | |
| Zinc | |
| Cyanide | |
| Chlorinated Hydrocarbons |
(1) L.T. McCarthy, Jr., I. Wilder, and J.S. Dorrier. Standard Dispersant Effectiveness and Toxicity Tests. EPA Report EPA-R2-73-201 (May 1973).
(2) M.F. Fingas, K.A. Hughes, and M.A. Schwertzer. “Dispersant Testing at the Environmental Emergencies Technology Division.” Proc. Tenth Arctic Marine Oilspill Program Technical Seminar. 9-11 June, 1987. Edmonton, Alberta, Canada. Conservation and Protection, Environment Canada. pp. 343-356.
(3) J.R. Clayton, Jr., S-F-Tsang, V. Frank, P. Marsden, and J. Harrington. Chemical Oil Spill Dispersants: Evaluation of Three Laboratory Procedures for Estimating Performance. Final report prepared by Science Applications International Corporation for U.S. Environmental Protection Agency, 1992.
(4) J.R. Clayton, Jr. and J.R. Payne. Chemical Oil Spill Dispersants: Update State-of-the-Art on Mechanisms of Actions and Factors Influencing Performance With Emphasis on Laboratory Studies. Final report prepared by Science Applications International Corporation for U.S. Environmental Protection Agency, 1992.
(5) D.P. Middaugh, M.J. Hemmer, and L. Goodman. Methods for Spawning, Cultureing and Conducting Toxicity-tests with Early Life Stages of Four Antherinid Fishes: the Inland Silverside, Menidia beryllina, Atlantic Silverside, M. menidia, Tidewater Silverside, M. penisulae, and California Grunion, Lesthes tenuis. Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC. EPA 600/8-87/004, 1987.
(6) U.S. EPA. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. Fourth edition. U.S. Environmental Protection Agency, Washington, D.C EPA 600/4-90/027, 1991.
(7) G.S. Douglas, et al. “The Use of Hydrocarbon Analyses for Environmental Assessment and Remediation.” In: P.T. Kostecki and E.J. Calabrese (eds.), Contaminated Soils, Diesel Fuel Contamination. Lewis Publishers, Ann Arbor, MI, 1992.
(8) Draft International Standard ISO/DIS 8708 “Crude Petroleum Oil - Determination of Distillation Characteristics Using 15 Theoretical Plates Columns.” International Organization for Standardization.
(9) Standard Methods for the Examination of Water and Wastewater, 17th Edition, American Public Health Association, 1989.
(10) U.S. EPA. Test Method for Evaluating Solid Waste: SW-846. Third edition. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, D.C, 1986.
(11) M.C. Kennicutt II. “The Effect of Bioremediation on Crude Oil Bulk and Molecular Composition.” In: Oil Chemical Pollution, 4:89-112, 1988.
(12) G.W. Snedecor and W.G. Cochran. Statistical Methods, 7th edition, The Iowa State University Press, Ames, Iowa, 1980.
(13) D.C. Montgomery. Design and Analysis of Experiments. Third edition. John Wiley & Sons, New York, NY, 1991.
[59 FR 47458, Sept. 15, 1994]