Title 20
PART 404 APPENDIX
| Calendar year | Amount needed |
|---|---|
| 1979 | $260 |
| 1980 | 290 |
| 1981 | 310 |
| 1982 | 340 |
| 1983 | 370 |
| 1984 | 390 |
| 1985 | 410 |
| 1986 | 440 |
| 1987 | 460 |
| 1988 | 470 |
| 1989 | 500 |
| 1990 | 520 |
| 1991 | 540 |
| 1992 | 570 |
Appendixes to Subpart C of Part 404 - Note
20:2.0.1.1.5.3.124.44.3 :
Appendixes to Subpart C of Part 404 - NoteThe following appendices contain data that are needed in computing primary insurance amounts. Appendix I contains average of the total wages figures, which we use to index a worker's earnings for purposes of computing his or her average indexed monthly earnings. Appendix II contains benefit formulas which we apply to a worker's average indexed monthly earnings to find his or her primary insurance amount. Appendix III contains the benefit table we use to find a worker's primary insurance amount from his or her average monthly wage. We use the figures in appendix IV to find your years of coverage for years after 1950 for purposes of your special minimum primary insurance amount. Appendix V contains the table for computing the special minimum primary insurance amount. Appendix VI is a table of the percentage increases in primary insurance amounts since 1978. Appendix VII is a table of the old-law contribution and benefit base that would have been effective under the Social Security Act without enactment of the 1977 amendments.
The figures in the appendices are by law automatically adjusted each year. We are required to announce the changes through timely publication in the Federal Register. The only exception to the requirement of publication in the Federal Register is the update of benefit amounts shown in appendix III. We update the benefit amounts for payment purposes but are not required by law to publish this extensive table in the Federal Register. We have not updated the table in appendix III, but the introductory paragraphs at appendix III explain how you can compute the current benefit amount.
When we publish the figures in the Federal Register, we do not change every one of these figures. Instead, we provide new ones for each year that passes. We continue to use the old ones for various computation purposes, as the regulations show. Most of the new figures for these appendices are required by law to be published by November 1 of each year. Notice of automatic cost-of-living increases in primary insurance amounts is required to be published within 45 days of the end of the applicable measuring period for the increase (see §§ 404.274 and 404.276). In effect, publication is required within 45 days of the end of the third calendar quarter of any year in which there is to be an automatic cost-of-living increase.
We begin to use the new data in computing primary insurance amounts as soon as required by law, even before we periodically update these appendices. If the data you need to find your primary insurance amount have not yet been included in the appendices, you may find the figures in the Federal Register on or about November 1.
[52 FR 8247, Mar. 17, 1987]Appendix I to Subpart C of Part 404 - Average of the Total Wages for Years After 1950
20:2.0.1.1.5.3.124.44.4 : Appendix I
Appendix I to Subpart C of Part 404 - Average of the Total Wages for Years After 1950Explanation: We use these figures to index your social security earnings (as described in § 404.211) for purposes of computing your average indexed monthly earnings.
| Calendar year | Average of the total wages |
|---|---|
| 1951 | $2,799.16 |
| 1952 | 2,973.32 |
| 1953 | 3,139.44 |
| 1954 | 3,155.64 |
| 1955 | 3,301.44 |
| 1956 | 3,532.36 |
| 1957 | 3,641.72 |
| 1958 | 3,673.80 |
| 1959 | 3,855.80 |
| 1960 | 4,007.12 |
| 1961 | 4,086.76 |
| 1962 | 4,291.40 |
| 1963 | 4,396.64 |
| 1964 | 4,576.32 |
| 1965 | 4,658.72 |
| 1966 | 4,938.36 |
| 1967 | 5,213.44 |
| 1968 | 5,571.76 |
| 1969 | 5,893.76 |
| 1970 | 6,186.24 |
| 1971 | 6,497.08 |
| 1972 | 7,133.80 |
| 1973 | 7,580.16 |
| 1974 | 8,030.76 |
| 1975 | 8,630.92 |
| 1976 | 9,226.48 |
| 1977 | 9,779.44 |
| 1978 | 10,556.03 |
| 1979 | 11,479.46 |
| 1980 | 12,513.46 |
| 1981 | 13,773.10 |
| 1982 | 14,531.34 |
| 1983 | 15,239.24 |
| 1984 | 16,135.07 |
| 1985 | 16,822.51 |
| 1986 | 17,321.82 |
| 1987 | 18,426.51 |
| 1988 | 19,334.04 |
| 1989 | 20,099.55 |
| 1990 | 21,027.98 |
Appendix II to Subpart C of Part 404 - Benefit Formulas Used With Average Indexed Monthly Earnings
20:2.0.1.1.5.3.124.44.5 : Appendix II
Appendix II to Subpart C of Part 404 - Benefit Formulas Used With Average Indexed Monthly EarningsAs explained in § 404.212, we use one of the formulas below to compute your primary insurance amount from your average indexed monthly earnings (AIME). To select the appropriate formula, we find in the left-hand column the year after 1978 in which you reach age 62, or become disabled, or die before age 62. The benefit formula to be used in computing your primary insurance amount is on the same line in the right-hand columns. For example, if you reach age 62 or become disabled or die before age 62 in 1979, then we compute 90 percent of the first $180 of AIME, 32 percent of the next $905 of AIME, and 15 percent of AIME over $1,085. After we figure your amount for each step in the formula, we add the amounts. If the total is not already a multiple of $0.10, we round the total as follows:
(1) For computations using the benefit formulas in effect for 1979 through 1982, we round the total upward to the nearest $0.10, and
(2) For computations using the benefit formulas in effect for 1983 and later, we round the total downward to the nearest $0.10.
Benefit Formulas
| Year you reach age 62 1 | 90 percent of the first - | plus 32 percent of the next - | plus 15 percent of AIME over - |
|---|---|---|---|
| 1979 | $180 | $905 | $1,085 |
| 1980 | 194 | 977 | 1,171 |
| 1981 | 211 | 1,063 | 1,274 |
| 1982 | 230 | 1,158 | 1,388 |
| 1983 | 254 | 1,274 | 1,528 |
| 1984 | 267 | 1,345 | 1,612 |
| 1985 | 280 | 1,411 | 1,691 |
| 1986 | 297 | 1,493 | 1,790 |
| 1987 | 310 | 1,556 | 1,866 |
| 1988 | 319 | 1,603 | 1,922 |
| 1989 | 339 | 1,705 | 2,044 |
| 1990 | 356 | 1,789 | 2,145 |
| 1991 | 370 | 1,860 | 2,230 |
| 1992 | 387 | 1,946 | 2,333 |
1 Or become disabled or die before age 62.
Appendix III to Subpart C of Part 404 - Benefit Table
20:2.0.1.1.5.3.124.44.6 : Appendix III
Appendix III to Subpart C of Part 404 - Benefit TableThis benefit table shows primary insurance amounts and maximum family benefits in effect in December 1978 based on cost-of-living increases which became effective for June 1978. (See § 404.403 for information on maximum family benefits.) You will also be able to find primary insurance amounts for an individual whose entitlement began in the period June 1977 through May 1978.
The benefit table in effect in December 1978 had a minimum primary insurance amount of $121.80. As explained in § 404.222(b), certain workers eligible, or who died without having been eligible, before 1982 had their benefit computed from this table. However, the minimum benefit provision was repealed for other workers by the 1981 amendments to the Act (the Omnibus Budget Reconciliation Act of 1981, Pub. L. 97-35 as modified by Pub. L. 97-123). As a result, this benefit table includes a downward extension from the former minimum of $121.80 to the lowest primary insurance amount now possible. The extension is calculated as follows. For each single dollar of average monthly wage in the benefit table, the primary insurance amount shown for December 1978 is $121.80 multiplied by the ratio of that average monthly wage to $76. The upper limit of each primary insurance benefit range in column I of the table is $16.20 multiplied by the ratio of the average monthly wage in column III of the table to $76. The maximum family benefit is 150 percent of the corresponding primary insurance amount.
The repeal of the minimum benefit provision is effective with January 1982 for most workers and their families where the worker initially becomes eligible for benefits after 1981 or dies after 1981 without having been eligible before January 1982. For members of a religious order who are required to take a vow of poverty, as explained in 20 CFR 404.1024, and which religious order elected Social Security coverage before December 29, 1981, the repeal is effective with January 1992 based on first eligibility or death in that month or later.
To use this table, you must first compute the primary insurance benefit (column I) or the average monthly wage (column III), then move across the same line to either column II or column IV as appropriate. To determine increases in primary insurance amounts since December 1978 you should see appendix VI. Appendix VI tells you, by year, the percentage of the increases. In applying each cost-of-living increase to primary insurance amounts, we round the increased primary insurance amount to the next lower multiple of $0.10 if not already a multiple of $0.10. (For cost-of-living increases which are effective before June 1982, we round to the next higher multiple of $0.10.)
Extended December 1978 Table of Benefits Effective January 1982
[In dollars]
| I. Primary insurance benefit: If an individual's primary insurance benefit (as determined under § 404.241(e)) is - | II. Primary insurance amount effective June 1977: Or his or her primary insurance amount is - | III. Average monthly wage: Or his or her average monthly wage (as determined under § 404.221) is - | IV. Primary insurance amount effective January 1982: Then his or her primary insurance amount is - | V. Maximum family benefits: And the maximum amount of benefits payable on the basis of his or her wages and self-employment income is - | ||
|---|---|---|---|---|---|---|
| At least - | But not more than - | At least - | But not more than - | |||
| 1 | 1.70 | 2.60 | ||||
| 0.42 | 2 | 2 | 3.30 | 5.00 | ||
| 0.43 | .63 | 3 | 3 | 4.90 | 7.40 | |
| .64 | .85 | 4 | 4 | 6.50 | 9.80 | |
| .86 | 1.06 | 5 | 5 | 8.10 | 12.20 | |
| 1.07 | 1.27 | 6 | 6 | 9.70 | 14.60 | |
| 1.28 | 1.49 | 7 | 7 | 11.30 | 17.00 | |
| 1.50 | 1.70 | 8 | 8 | 12.90 | 19.40 | |
| 1.71 | 1.91 | 9 | 9 | 14.50 | 21.80 | |
| 1.92 | 2.13 | 10 | 10 | 16.10 | 24.20 | |
| 2.14 | 2.34 | 11 | 11 | 17.70 | 26.60 | |
| 2.35 | 2.55 | 12 | 12 | 19.30 | 29.00 | |
| 2.56 | 2.77 | 13 | 13 | 20.90 | 31.40 | |
| 2.78 | 2.98 | 14 | 14 | 22.50 | 33.80 | |
| 2.99 | 3.19 | 15 | 15 | 24.10 | 36.20 | |
| 3.20 | 3.41 | 16 | 16 | 25.70 | 38.60 | |
| 3.42 | 3.62 | 17 | 17 | 27.30 | 41.00 | |
| 3.63 | 3.83 | 18 | 18 | 28.90 | 43.40 | |
| 3.84 | 4.05 | 19 | 19 | 30.50 | 45.80 | |
| 4.06 | 4.26 | 20 | 20 | 32.10 | 48.20 | |
| 4.27 | 4.47 | 21 | 21 | 33.70 | 50.60 | |
| 4.48 | 4.68 | 22 | 22 | 35.30 | 53.00 | |
| 4.69 | 4.90 | 23 | 23 | 36.90 | 55.40 | |
| 4.91 | 5.11 | 24 | 24 | 38.50 | 57.80 | |
| 5.12 | 5.32 | 25 | 25 | 40.10 | 60.20 | |
| 5.33 | 5.54 | 26 | 26 | 41.70 | 62.60 | |
| 5.55 | 5.75 | 27 | 27 | 43.30 | 65.00 | |
| 5.76 | 5.96 | 28 | 28 | 44.90 | 67.40 | |
| 5.97 | 6.18 | 29 | 29 | 46.50 | 69.80 | |
| 6.19 | 6.39 | 30 | 30 | 48.10 | 72.20 | |
| 6.40 | 6.60 | 31 | 31 | 49.70 | 74.60 | |
| 6.61 | 6.82 | 32 | 32 | 51.30 | 77.00 | |
| 6.83 | 7.03 | 33 | 33 | 52.90 | 79.40 | |
| 7.04 | 7.24 | 34 | 34 | 54.50 | 81.80 | |
| 7.25 | 7.46 | 35 | 35 | 56.10 | 84.20 | |
| 7.47 | 7.67 | 36 | 36 | 57.70 | 86.60 | |
| 7.68 | 7.88 | 37 | 37 | 59.30 | 89.00 | |
| 7.89 | 8.10 | 38 | 38 | 60.90 | 91.40 | |
| 8.11 | 8.31 | 39 | 39 | 62.60 | 93.90 | |
| 8.32 | 8.52 | 40 | 40 | 64.20 | 96.30 | |
| 8.53 | 8.73 | 41 | 41 | 65.80 | 98.70 | |
| 8.74 | 8.95 | 42 | 42 | 67.40 | 101.10 | |
| 8.96 | 9.16 | 43 | 43 | 69.00 | 103.50 | |
| 9.17 | 9.37 | 44 | 44 | 70.60 | 105.90 | |
| 9.38 | 9.59 | 45 | 45 | 72.20 | 108.30 | |
| 9.60 | 9.80 | 46 | 46 | 73.80 | 110.70 | |
| 9.81 | 10.01 | 47 | 47 | 75.40 | 113.10 | |
| 10.02 | 10.23 | 48 | 48 | 77.00 | 115.50 | |
| 10.24 | 10.44 | 49 | 49 | 78.60 | 117.90 | |
| 10.45 | 10.65 | 50 | 50 | 80.20 | 120.30 | |
| 10.66 | 10.87 | 51 | 51 | 81.80 | 122.70 | |
| 10.88 | 11.08 | 52 | 52 | 83.40 | 125.10 | |
| 11.09 | 11.29 | 53 | 53 | 85.00 | 127.50 | |
| 11.30 | 11.51 | 54 | 54 | 86.60 | 129.90 | |
| 11.52 | 11.72 | 55 | 55 | 88.20 | 132.30 | |
| 11.73 | 11.93 | 56 | 56 | 89.80 | 134.70 | |
| 11.94 | 12.15 | 57 | 57 | 91.40 | 137.10 | |
| 12.16 | 12.36 | 58 | 58 | 93.00 | 139.50 | |
| 12.37 | 12.57 | 59 | 59 | 94.60 | 141.90 | |
| 12.58 | 12.78 | 60 | 60 | 96.20 | 144.30 | |
| 12.79 | 13.00 | 61 | 61 | 97.80 | 146.70 | |
| 13.01 | 13.21 | 62 | 62 | 99.40 | 149.10 | |
| 13.22 | 13.42 | 63 | 63 | 101.00 | 151.50 | |
| 13.43 | 13.64 | 64 | 64 | 102.60 | 153.90 | |
| 13.65 | 13.85 | 65 | 65 | 104.20 | 156.30 | |
| 13.86 | 14.06 | 66 | 66 | 105.80 | 158.70 | |
| 14.07 | 14.28 | 67 | 67 | 107.40 | 161.10 | |
| 14.29 | 14.49 | 68 | 68 | 109.00 | 163.50 | |
| 14.50 | 14.70 | 69 | 69 | 110.60 | 165.90 | |
| 14.71 | 14.92 | 70 | 70 | 112.20 | 168.30 | |
| 14.93 | 15.13 | 71 | 71 | 113.80 | 170.70 | |
| 15.14 | 15.34 | 72 | 72 | 115.40 | 173.10 | |
| 15.35 | 15.56 | 73 | 73 | 117.00 | 175.50 | |
| 15.57 | 15.77 | 74 | 74 | 118.60 | 177.90 | |
| 15.78 | 15.98 | 75 | 75 | 120.20 | 180.30 | |
| 15.99 | 16.20 | 76 | 76 | 121.80 | 182.70 | |
Table of Benefits in Effect in December 1978
[In dollars]
| I. Primary insurance benefit: If an individual's primary insurance benefit (as determined under § 404.241(e)) is - | II. Primary insurance amount effective June 1977: Or his or her primary insurance amount is - | III. Average monthly wage: Or his or her average monthly wage (as determined under § 404.221) is - | IV. Primary insurance amount effective June 1978: Then his or her primary insurance amount is - | V. Maximum family benefits: And the maximum amount of benefits payable on the basis of his or her wages and self-employment income is - | ||
|---|---|---|---|---|---|---|
| At least - | But not more than - | At least - | But not more than - | |||
| 16.20 | 114.30 | 76 | 121.80 | 182.70 | ||
| 16.21 | 16.84 | 116.10 | 77 | 78 | 123.70 | 185.60 |
| 16.85 | 17.60 | 118.80 | 79 | 80 | 126.60 | 189.90 |
| 17.61 | 18.40 | 121.00 | 81 | 81 | 128.90 | 193.50 |
| 18.41 | 19.24 | 123.00 | 82 | 83 | 131.20 | 196.80 |
| 19.25 | 20.00 | 125.80 | 84 | 85 | 134.00 | 201.00 |
| 20.01 | 20.64 | 128.10 | 86 | 87 | 136.50 | 204.80 |
| 20.65 | 21.28 | 130.10 | 88 | 89 | 138.60 | 207.90 |
| 21.29 | 21.88 | 132.70 | 90 | 90 | 141.40 | 212.10 |
| 21.89 | 22.28 | 135.00 | 91 | 92 | 143.80 | 215.70 |
| 22.29 | 22.68 | 137.20 | 93 | 94 | 146.20 | 219.20 |
| 22.59 | 23.08 | 139.40 | 95 | 96 | 148.50 | 222.80 |
| 23.09 | 23.44 | 142.00 | 97 | 97 | 151.30 | 227.00 |
| 23.45 | 23.76 | 144.30 | 98 | 99 | 153.70 | 230.60 |
| 23.77 | 24.20 | 147.10 | 100 | 101 | 156.70 | 235.10 |
| 24.21 | 24.60 | 149.20 | 102 | 102 | 158.90 | 238.50 |
| 24.61 | 25.00 | 151.70 | 103 | 104 | 161.60 | 242.40 |
| 25.01 | 25.48 | 154.50 | 105 | 106 | 164.60 | 246.90 |
| 25.49 | 25.92 | 157.00 | 107 | 107 | 167.30 | 251.00 |
| 25.93 | 26.40 | 159.40 | 108 | 109 | 169.80 | 254.80 |
| 26.41 | 26.94 | 161.90 | 110 | 113 | 172.50 | 258.80 |
| 26.95 | 27.46 | 164.20 | 114 | 118 | 174.90 | 262.40 |
| 27.47 | 28.00 | 166.70 | 119 | 122 | 177.60 | 266.50 |
| 28.01 | 28.68 | 169.30 | 123 | 127 | 180.40 | 270.60 |
| 28.69 | 29.25 | 171.80 | 128 | 132 | 183.00 | 274.60 |
| 29.26 | 29.68 | 174.10 | 133 | 136 | 185.50 | 278.30 |
| 29.69 | 30.36 | 176.50 | 137 | 141 | 188.00 | 282.10 |
| 30.37 | 30.92 | 179.10 | 142 | 146 | 190.80 | 286.20 |
| 30.93 | 31.36 | 181.70 | 147 | 150 | 193.60 | 290.40 |
| 31.37 | 32.00 | 183.90 | 151 | 155 | 195.90 | 293.90 |
| 32.01 | 32.60 | 186.50 | 156 | 160 | 198.70 | 298.10 |
| 32.61 | 33.20 | 189.00 | 161 | 164 | 201.30 | 302.00 |
| 33.21 | 33.88 | 191.40 | 165 | 169 | 203.90 | 305.90 |
| 33.89 | 34.50 | 194.00 | 170 | 174 | 206.70 | 310.10 |
| 34.51 | 35.00 | 196.30 | 175 | 178 | 209.10 | 313.70 |
| 35.01 | 35.80 | 198.90 | 179 | 183 | 211.90 | 318.00 |
| 35.81 | 36.40 | 201.30 | 184 | 188 | 214.40 | 321.70 |
| 36.41 | 37.08 | 203.90 | 189 | 193 | 217.20 | 326.00 |
| 37.09 | 37.60 | 206.40 | 194 | 197 | 219.90 | 329.90 |
| 37.61 | 38.20 | 208.80 | 198 | 202 | 222.40 | 333.60 |
| 38.21 | 39.12 | 211.50 | 203 | 207 | 225.30 | 338.00 |
| 39.13 | 39.68 | 214.00 | 208 | 211 | 228.00 | 342.00 |
| 39.69 | 40.33 | 216.00 | 212 | 216 | 230.10 | 345.20 |
| 40.34 | 41.12 | 218.70 | 217 | 221 | 233.00 | 349.50 |
| 41.13 | 41.76 | 221.20 | 222 | 225 | 235.60 | 353.40 |
| 41.77 | 42.44 | 223.90 | 226 | 230 | 238.50 | 357.80 |
| 42.45 | 43.20 | 226.30 | 231 | 235 | 241.10 | 361.70 |
| 43.21 | 43.76 | 229.10 | 236 | 239 | 244.00 | 366.10 |
| 43.77 | 44.44 | 231.20 | 240 | 244 | 246.30 | 371.10 |
| 44.45 | 44.88 | 233.50 | 245 | 249 | 248.70 | 378.80 |
| 44.89 | 45.60 | 236.40 | 250 | 253 | 251.80 | 384.90 |
| 238.70 | 254 | 258 | 254.30 | 392.50 | ||
| 240.80 | 259 | 263 | 256.50 | 400.00 | ||
| 243.70 | 264 | 267 | 259.60 | 206.00 | ||
| 246.10 | 268 | 272 | 262.10 | 413.70 | ||
| 248.70 | 273 | 277 | 264.90 | 421.20 | ||
| 251.00 | 278 | 281 | 267.40 | 427.20 | ||
| 253.50 | 282 | 286 | 270.00 | 434.90 | ||
| 256.20 | 287 | 291 | 272.90 | 442.60 | ||
| 258.30 | 292 | 295 | 275.10 | 448.50 | ||
| 261.10 | 296 | 300 | 278.10 | 456.10 | ||
| 263.50 | 301 | 305 | 280.70 | 463.80 | ||
| 265.80 | 306 | 309 | 283.10 | 469.80 | ||
| 268.50 | 310 | 314 | 286.00 | 477.40 | ||
| 270.70 | 315 | 319 | 288.30 | 485.10 | ||
| 273.20 | 320 | 323 | 291.00 | 491.10 | ||
| 275.80 | 324 | 328 | 293.80 | 498.70 | ||
| 278.10 | 329 | 333 | 296.20 | 506.20 | ||
| 281.00 | 334 | 337 | 299.30 | 512.50 | ||
| 283.00 | 338 | 342 | 301.40 | 519.90 | ||
| 285.60 | 343 | 347 | 304.20 | 527.50 | ||
| 288.30 | 348 | 351 | 307.10 | 533.60 | ||
| 290.50 | 352 | 356 | 309.40 | 541.20 | ||
| 293.30 | 357 | 361 | 312.40 | 548.80 | ||
| 295.60 | 362 | 365 | 314.90 | 554.90 | ||
| 297.90 | 366 | 370 | 317.30 | 562.50 | ||
| 300.60 | 371 | 375 | 320.20 | 569.90 | ||
| 303.10 | 376 | 379 | 322.90 | 576.30 | ||
| 305.70 | 380 | 384 | 325.60 | 583.90 | ||
| 307.90 | 385 | 389 | 328.00 | 591.30 | ||
| 310.30 | 390 | 393 | 330.50 | 597.40 | ||
| 313.00 | 394 | 398 | 333.40 | 605.10 | ||
| 315.40 | 399 | 403 | 336.00 | 612.70 | ||
| 318.20 | 404 | 407 | 338.90 | 618.60 | ||
| 320.20 | 408 | 412 | 341.10 | 626.30 | ||
| 322.50 | 413 | 417 | 343.50 | 633.80 | ||
| 324.80 | 418 | 421 | 346.00 | 639.90 | ||
| 327.40 | 422 | 426 | 348.70 | 647.50 | ||
| 329.60 | 427 | 431 | 351.10 | 655.10 | ||
| 331.60 | 432 | 436 | 353.20 | 662.70 | ||
| 334.40 | 437 | 440 | 356.20 | 665.70 | ||
| 336.50 | 441 | 445 | 358.40 | 669.70 | ||
| 338.70 | 446 | 450 | 360.80 | 673.40 | ||
| 341.30 | 451 | 454 | 363.50 | 676.30 | ||
| 343.50 | 455 | 459 | 365.90 | 680.10 | ||
| 345.80 | 460 | 464 | 368.30 | 683.80 | ||
| 347.90 | 465 | 468 | 370.60 | 687.10 | ||
| 350.70 | 469 | 473 | 373.50 | 690.80 | ||
| 352.60 | 474 | 478 | 375.60 | 694.60 | ||
| 354.90 | 479 | 482 | 378.00 | 697.70 | ||
| 357.40 | 483 | 487 | 380.70 | 701.60 | ||
| 359.70 | 488 | 492 | 383.10 | 705.40 | ||
| 361.90 | 493 | 496 | 385.50 | 708.40 | ||
| 364.50 | 497 | 501 | 388.20 | 712.10 | ||
| 366.60 | 502 | 506 | 390.50 | 715.80 | ||
| 368.90 | 507 | 510 | 392.90 | 719.00 | ||
| 371.10 | 511 | 515 | 395.30 | 722.80 | ||
| 373.70 | 516 | 520 | 398.00 | 726.70 | ||
| 375.80 | 521 | 524 | 400.30 | 729.50 | ||
| 378.10 | 525 | 529 | 402.70 | 733.40 | ||
| 380.80 | 530 | 534 | 405.60 | 737.10 | ||
| 382.80 | 535 | 538 | 407.70 | 740.20 | ||
| 385.10 | 539 | 543 | 410.20 | 744.10 | ||
| 387.60 | 544 | 548 | 412.80 | 747.80 | ||
| 389.90 | 549 | 553 | 415.30 | 751.60 | ||
| 392.10 | 554 | 556 | 417.60 | 753.90 | ||
| 393.90 | 557 | 560 | 419.60 | 756.90 | ||
| 396.10 | 561 | 563 | 421.90 | 759.30 | ||
| 398.20 | 564 | 567 | 424.10 | 762.30 | ||
| 400.40 | 568 | 570 | 426.50 | 764.50 | ||
| 402.30 | 571 | 574 | 428.50 | 767.50 | ||
| 404.40 | 575 | 577 | 430.70 | 769.90 | ||
| 406.20 | 578 | 581 | 432.70 | 772.80 | ||
| 408.40 | 582 | 584 | 435.00 | 775.20 | ||
| 410.20 | 585 | 588 | 436.90 | 778.20 | ||
| 412.60 | 589 | 591 | 439.50 | 780.50 | ||
| 414.60 | 592 | 595 | 441.60 | 783.50 | ||
| 416.70 | 596 | 598 | 443.80 | 785.60 | ||
| 418.70 | 599 | 602 | 446.00 | 788.90 | ||
| 420.70 | 603 | 605 | 448.10 | 791.10 | ||
| 422.80 | 606 | 609 | 450.30 | 794.00 | ||
| 424.90 | 610 | 612 | 452.60 | 796.50 | ||
| 426.90 | 613 | 616 | 454.70 | 799.50 | ||
| 428.90 | 617 | 620 | 456.80 | 802.50 | ||
| 431.00 | 621 | 623 | 459.10 | 804.80 | ||
| 433.00 | 624 | 627 | 461.20 | 807.90 | ||
| 435.10 | 628 | 630 | 463.40 | 810.70 | ||
| 437.10 | 631 | 634 | 465.60 | 814.70 | ||
| 439.20 | 635 | 637 | 467.80 | 818.50 | ||
| 441.40 | 638 | 641 | 470.10 | 822.40 | ||
| 443.20 | 642 | 644 | 472.10 | 826.10 | ||
| 445.40 | 645 | 648 | 474.40 | 830.10 | ||
| 447.40 | 649 | 652 | 476.50 | 833.70 | ||
| 448.60 | 653 | 656 | 477.80 | 836.10 | ||
| 449.90 | 657 | 660 | 479.20 | 838.40 | ||
| 451.50 | 661 | 665 | 480.90 | 841.50 | ||
| 453.10 | 666 | 670 | 482.60 | 844.50 | ||
| 454.80 | 671 | 675 | 484.40 | 847.40 | ||
| 456.40 | 676 | 680 | 486.10 | 850.50 | ||
| 458.00 | 681 | 685 | 487.80 | 853.50 | ||
| 459.80 | 686 | 690 | 489.70 | 856.40 | ||
| 461.20 | 691 | 695 | 491.20 | 859.60 | ||
| 462.80 | 696 | 700 | 492.90 | 862.60 | ||
| 464.50 | 701 | 705 | 494.70 | 865.60 | ||
| 466.10 | 706 | 710 | 496.40 | 868.60 | ||
| 467.70 | 711 | 715 | 498.20 | 871.50 | ||
| 469.40 | 716 | 720 | 500.00 | 874.60 | ||
| 471.00 | 721 | 725 | 501.70 | 877.60 | ||
| 472.60 | 726 | 730 | 503.40 | 880.70 | ||
| 474.20 | 731 | 735 | 505.10 | 883.80 | ||
| 475.90 | 736 | 740 | 506.90 | 886.70 | ||
| 477.40 | 741 | 745 | 508.50 | 889.90 | ||
| 478.90 | 746 | 750 | 510.10 | 892.70 | ||
| 480.40 | 751 | 755 | 511.70 | 896.40 | ||
| 481.80 | 756 | 760 | 513.20 | 897.80 | ||
| 483.20 | 761 | 765 | 514.70 | 900.40 | ||
| 484.50 | 766 | 770 | 516.00 | 903.00 | ||
| 485.80 | 771 | 775 | 517.40 | 905.40 | ||
| 487.20 | 776 | 780 | 518.90 | 907.90 | ||
| 488.60 | 781 | 785 | 520.40 | 910.40 | ||
| 489.80 | 786 | 790 | 521.70 | 912.90 | ||
| 491.10 | 791 | 795 | 523.10 | 915.40 | ||
| 492.50 | 796 | 800 | 524.60 | 918.00 | ||
| 494.00 | 801 | 805 | 526.20 | 920.50 | ||
| 495.30 | 806 | 810 | 527.50 | 923.00 | ||
| 496.70 | 811 | 815 | 529.00 | 925.60 | ||
| 498.00 | 816 | 820 | 530.40 | 928.00 | ||
| 499.40 | 821 | 825 | 531.90 | 930.60 | ||
| 500.70 | 826 | 830 | 533.30 | 933.10 | ||
| 502.00 | 831 | 835 | 534.70 | 935.70 | ||
| 503.30 | 836 | 840 | 536.10 | 938.10 | ||
| 504.70 | 841 | 845 | 537.60 | 940.80 | ||
| 506.00 | 846 | 850 | 538.90 | 943.00 | ||
| 507.50 | 851 | 855 | 540.50 | 945.70 | ||
| 508.80 | 856 | 860 | 541.90 | 948.10 | ||
| 510.20 | 861 | 865 | 543.40 | 950.70 | ||
| 511.50 | 866 | 870 | 544.80 | 953.20 | ||
| 512.90 | 871 | 875 | 546.30 | 955.70 | ||
| 514.10 | 876 | 880 | 547.60 | 958.20 | ||
| 515.50 | 881 | 885 | 549.10 | 960.80 | ||
| 516.80 | 886 | 890 | 550.40 | 963.20 | ||
| 518.20 | 891 | 895 | 551.90 | 966.00 | ||
| 519.60 | 896 | 900 | 553.40 | 968.30 | ||
| 521.00 | 901 | 905 | 554.90 | 970.90 | ||
| 522.30 | 906 | 910 | 556.30 | 973.50 | ||
| 523.70 | 911 | 915 | 557.80 | 976.00 | ||
| 525.10 | 916 | 920 | 559.30 | 978.30 | ||
| 526.30 | 921 | 925 | 560.60 | 961.00 | ||
| 527.60 | 926 | 930 | 561.90 | 983.40 | ||
| 529.00 | 931 | 935 | 563.40 | 985.90 | ||
| 530.40 | 936 | 940 | 564.90 | 988.50 | ||
| 531.70 | 941 | 945 | 566.30 | 991.00 | ||
| 533.00 | 946 | 950 | 567.70 | 993.50 | ||
| 534.50 | 951 | 955 | 569.30 | 996.10 | ||
| 535.90 | 956 | 960 | 570.80 | 998.60 | ||
| 537.30 | 961 | 965 | 572.30 | 1,001.00 | ||
| 538.40 | 966 | 970 | 573.40 | 1,003.60 | ||
| 539.80 | 971 | 975 | 574.90 | 1,006.20 | ||
| 541.20 | 976 | 980 | 576.40 | 1,008.50 | ||
| 542.60 | 981 | 985 | 577.90 | 1,011.10 | ||
| 543.80 | 986 | 990 | 579.20 | 1,013.60 | ||
| 545.20 | 991 | 995 | 580.70 | 1,016.20 | ||
| 546.60 | 996 | 1,000 | 582.20 | 1,018.60 | ||
| 547.80 | 1,001 | 1,005 | 583.50 | 1,020.70 | ||
| 548.90 | 1,006 | 1,010 | 584.60 | 1,023.20 | ||
| 550.20 | 1,011 | 1,015 | 586.00 | 1,025.30 | ||
| 551.50 | 1,016 | 1,020 | 587.40 | 1,027.80 | ||
| 552.60 | 1,021 | 1,025 | 588.60 | 1,029.90 | ||
| 553.80 | 1,026 | 1,030 | 589.80 | 1,032.20 | ||
| 555.10 | 1,031 | 1,035 | 591.20 | 1,034.50 | ||
| 556.20 | 1,036 | 1,040 | 592.40 | 1,036.70 | ||
| 557.50 | 1,041 | 1,045 | 593.80 | 1,039.10 | ||
| 558.80 | 1,046 | 1,050 | 595.20 | 1,041.30 | ||
| 559.80 | 1,051 | 1,055 | 596.20 | 1,043.40 | ||
| 561.10 | 1,056 | 1,060 | 597.60 | 1,045.90 | ||
| 562.40 | 1,061 | 1,065 | 599.00 | 1,048.00 | ||
| 563.60 | 1,066 | 1,070 | 600.30 | 1,050.50 | ||
| 564.80 | 1,071 | 1,075 | 601.60 | 1,052.60 | ||
| 566.00 | 1,076 | 1,080 | 602.80 | 1,054.90 | ||
| 567.30 | 1,081 | 1,085 | 604.20 | 1,057.10 | ||
| 568.40 | 1,086 | 1,090 | 605.40 | 1,059.40 | ||
| 569.70 | 1,091 | 1,095 | 606.80 | 1,061.70 | ||
| 571.00 | 1,096 | 1,100 | 608.20 | 1,064.00 | ||
| 572.00 | 1,101 | 1,105 | 609.20 | 1,066.10 | ||
| 573.30 | 1,106 | 1,110 | 610.60 | 1.068.50 | ||
| 574.60 | 1,111 | 1,115 | 612.00 | 1,070.70 | ||
| 575.70 | 1,116 | 1,120 | 613.20 | 1,073.10 | ||
| 577.00 | 1,121 | 1,125 | 614.60 | 1,075.30 | ||
| 578.20 | 1,126 | 1,130 | 615.80 | 1,077.60 | ||
| 579.40 | 1,131 | 1,135 | 617.10 | 1,079.70 | ||
| 580.60 | 1,136 | 1,140 | 618.40 | 1,082.20 | ||
| 581.90 | 1,141 | 1,145 | 619.80 | 1,084.40 | ||
| 583.10 | 1,146 | 1,150 | 621.10 | 1,086.70 | ||
| 584.20 | 1,151 | 1,555 | 622.20 | 1,088.80 | ||
| 585.50 | 1,156 | 1,160 | 623.60 | 1,091.10 | ||
| 586.70 | 1,161 | 1,165 | 624.90 | 1,093.40 | ||
| 587.90 | 1,166 | 1,170 | 626.20 | 1,095.80 | ||
| 589.20 | 1,171 | 1,175 | 627.50 | 1,098.00 | ||
| 590.30 | 1,176 | 1,180 | 628.70 | 1,100.20 | ||
| 591.40 | 1,181 | 1,185 | 629.90 | 1,102.20 | ||
| 592.60 | 1,186 | 1,190 | 631.20 | 1,104.30 | ||
| 593.70 | 1,191 | 1,195 | 632.30 | 1,106.50 | ||
| 594.80 | 1,196 | 1,200 | 633.50 | 1,108.60 | ||
| 595.90 | 1,201 | 1,205 | 634.70 | 1,110.60 | ||
| 597.10 | 1,206 | 1,210 | 636.00 | 1,112.90 | ||
| 598.20 | 1,211 | 1,215 | 637.10 | 1,114.90 | ||
| 599.30 | 1,216 | 1,220 | 638.30 | 1,117.00 | ||
| 600.40 | 1,221 | 1,225 | 639.50 | 1,119.00 | ||
| 601.60 | 1,226 | 1,230 | 640.80 | 1,121.20 | ||
| 602.70 | 1,231 | 1,235 | 641.90 | 1,123.30 | ||
| 603.80 | 1,236 | 1,240 | 643.10 | 1,125.40 | ||
| 605.00 | 1,241 | 1,245 | 644.40 | 1,127.50 | ||
| 606.10 | 1,246 | 1,250 | 645.50 | 1,129.60 | ||
| 607.20 | 1,251 | 1,255 | 646.70 | 1,131.60 | ||
| 608.30 | 1,256 | 1,260 | 647.90 | 1,133.80 | ||
| 609.50 | 1,261 | 1,265 | 649.20 | 1,135.90 | ||
| 610.60 | 1,266 | 1,270 | 650.30 | 1,138.00 | ||
| 611.70 | 1,271 | 1,275 | 651.50 | 1,140.00 | ||
| 612.80 | 1,276 | 1,280 | 652.70 | 1,142.20 | ||
| 613.80 | 1,281 | 1,285 | 653.70 | 1,144.10 | ||
| 614.80 | 1,286 | 1,290 | 654.90 | 1,146.10 | ||
| 616.00 | 1,291 | 1,295 | 656.10 | 1,148.00 | ||
| 617.00 | 1,296 | 1,300 | 657.20 | 1,150.00 | ||
| 618.10 | 1,301 | 1,305 | 658.30 | 1,152.00 | ||
| 619.10 | 1,306 | 1,310 | 659.40 | 1,154.00 | ||
| 620.20 | 1,311 | 1,315 | 660.60 | 1,155.90 | ||
| 621.30 | 1,316 | 1,320 | 661.70 | 1,157.90 | ||
| 622.30 | 1,321 | 1,325 | 662.80 | 1,159.80 | ||
| 623.40 | 1,326 | 1,330 | 664.00 | 1,161.90 | ||
| 624.40 | 1,331 | 1,335 | 665.00 | 1,163.80 | ||
| 625.50 | 1,336 | 1,340 | 666.20 | 1,165.80 | ||
| 626.60 | 1,341 | 1,345 | 667.40 | 1,167.70 | ||
| 627.60 | 1,346 | 1,350 | 668.40 | 1,169.70 | ||
| 628.70 | 1,351 | 1,355 | 669.60 | 1,171.70 | ||
| 629.70 | 1,356 | 1,360 | 670.70 | 1,173.70 | ||
| 630.80 | 1,361 | 1,365 | 671.90 | 1,175.60 | ||
| 631.80 | 1,366 | 1,370 | 672.90 | 1,177.70 | ||
| 632.90 | 1,371 | 1,375 | 674.10 | 1,179.60 | ||
| 633.90 | 1,376 | 1,380 | 675.20 | 1,181.60 | ||
| 634.90 | 1,381 | 1,385 | 676.20 | 1,183.40 | ||
| 635.90 | 1,386 | 1,390 | 677.30 | 1,185.30 | ||
| 636.90 | 1,391 | 1,395 | 678.30 | 1,187.10 | ||
| 637.90 | 1,396 | 1,400 | 679.40 | 1,189.00 | ||
| 638.90 | 1,401 | 1,405 | 680.50 | 1,190.80 | ||
| 639.90 | 1,406 | 1,410 | 681.50 | 1,192.70 | ||
| 640.90 | 1,411 | 1,415 | 682.60 | 1,194.60 | ||
| 641.90 | 1,416 | 1,420 | 683.70 | 1,196.50 | ||
| 642.90 | 1,421 | 1,425 | 685.70 | 1,198.30 | ||
| 643.90 | 1,426 | 1,430 | 684.80 | 1,200.20 | ||
| 644.90 | 1,431 | 1,435 | 686.90 | 1,202.00 | ||
| 645.90 | 1,436 | 1,440 | 687.90 | 1,203.90 | ||
| 646.90 | 1,441 | 1,445 | 689.00 | 1,205.70 | ||
| 647.90 | 1,446 | 1,450 | 690.10 | 1,207.70 | ||
| 648.90 | 1,451 | 1,455 | 691.10 | 1,209.50 | ||
| 649.90 | 1,456 | 1,460 | 692.20 | 1,211.40 | ||
| 650.90 | 1,461 | 1,465 | 693.30 | 1,213.20 | ||
| 651.90 | 1,466 | 1,470 | 694.30 | 1,215.10 | ||
| 652.90 | 1,471 | 1,475 | 695.40 | 1,216.90 | ||
Appendix IV to Subpart C of Part 404 - Earnings Needed for a Year of Coverage After 1950
20:2.0.1.1.5.3.124.44.7 : Appendix IV
Appendix IV to Subpart C of Part 404 - Earnings Needed for a Year of Coverage After 1950Minimum Social Security Earnings to Qualify for a Year of Coverage After 1950 for Purposes of the -
| Year | Special minimum primary insurance amount | Benefit computations described in section 404.213(d) 2 |
|---|---|---|
| 1951-1954 | $900 | $900 |
| 1955-1958 | 1,050 | 1,050 |
| 1959-1965 | 1,200 | 1,200 |
| 1966-1967 | 1,650 | 1,650 |
| 1968-1971 | 1,950 | 1,950 |
| 1972 | 2,250 | 2,250 |
| 1973 | 2,700 | 2,700 |
| 1974 | 3,300 | 3,300 |
| 1975 | 3,525 | 3,525 |
| 1976 | 3,825 | 3,825 |
| 1977 | 4,125 | 4,125 |
| 1978 | 4,425 | 4,425 |
| 1979 | 4,725 | 4,725 |
| 1980 | 5,100 | 5,100 |
| 1981 | 5,550 | 5,550 |
| 1982 | 6,075 | 6,075 |
| 1983 | 6,675 | 6,675 |
| 1984 | 7,050 | 7,050 |
| 1985 | 7,425 | 7,425 |
| 1986 | 7,875 | 7,875 |
| 1987 | 8,175 | 8,175 |
| 1988 | 8,400 | 8,400 |
| 1989 | 8,925 | 8,925 |
| 1990 | 9,525 | 9,525 |
| 1991 | 5,940 | 9,900 |
| 1992 | 6,210 | 10,350 |
2 Applies only to certain individuals with pensions from noncovered employment.
For 1951-78, the amounts shown are 25 percent of the contribution and benefit base (the contribution and benefit base is the same as the annual wage limitation as shown in § 404.1047) in effect. For years after 1978, however, the amounts are 25 percent of what the contribution and benefit base would have been if the 1977 Social Security Amendments had not been enacted, except, for special minimum benefit purposes, the applicable percentage is 15 percent for years after 1990.
[57 FR 44096, Sept. 24, 1992]Appendix V to Subpart C of Part 404 - Computing the Special Minimum Primary Insurance Amount and Related Maximum Family Benefits
20:2.0.1.1.5.3.124.44.8 : Appendix V
Appendix V to Subpart C of Part 404 - Computing the Special Minimum Primary Insurance Amount and Related Maximum Family BenefitsThese tables are based on section 215(a)(1)(C)(i) of the Social Security Act, as amended. They include the percent cost-of-living increase shown in appendix VI for each effective date.
June 1979
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $12.70 | $19.10 |
| 12 | 25.30 | 38.00 |
| 13 | 38.00 | 57.00 |
| 14 | 50.60 | 75.90 |
| 15 | 63.20 | 94.90 |
| 16 | 75.90 | 113.90 |
| 17 | 88.50 | 132.80 |
| 18 | 101.20 | 151.80 |
| 19 | 113.80 | 170.70 |
| 20 | 126.40 | 189.60 |
| 21 | 139.10 | 208.70 |
| 22 | 151.70 | 227.60 |
| 23 | 164.40 | 246.60 |
| 24 | 177.00 | 265.50 |
| 25 | 189.60 | 284.50 |
| 26 | 202.30 | 303.50 |
| 27 | 214.90 | 322.40 |
| 28 | 227.50 | 341.30 |
| 29 | 240.20 | 360.30 |
| 30 | 252.80 | 379.20 |
June 1980
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $14.60 | $21.90 |
| 12 | 29.00 | 43.50 |
| 13 | 43.50 | 65.30 |
| 14 | 57.90 | 86.90 |
| 15 | 72.30 | 108.50 |
| 16 | 86.80 | 130.20 |
| 17 | 101.20 | 151.80 |
| 18 | 115.70 | 173.60 |
| 19 | 130.10 | 195.20 |
| 20 | 144.50 | 216.80 |
| 21 | 159.00 | 238.60 |
| 22 | 173.40 | 260.20 |
| 23 | 188.00 | 282.00 |
| 24 | 202.40 | 303.60 |
| 25 | 216.80 | 325.20 |
| 26 | 231.30 | 347.00 |
| 27 | 245.70 | 368.60 |
| 28 | 260.10 | 390.20 |
| 29 | 274.60 | 411.90 |
| 30 | 289.00 | 433.50 |
June 1981
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefits |
|---|---|---|
| 11 | $16.30 | $24.50 |
| 12 | 32.30 | 48.50 |
| 13 | 48.40 | 72.70 |
| 14 | 64.40 | 96.70 |
| 15 | 80.40 | 120.70 |
| 16 | 96.60 | 144.90 |
| 17 | 112.60 | 168.90 |
| 18 | 128.70 | 193.10 |
| 19 | 144.70 | 217.10 |
| 20 | 160.70 | 241.10 |
| 21 | 176.90 | 265.40 |
| 22 | 192.90 | 289.40 |
| 23 | 209.10 | 313.70 |
| 24 | 225.10 | 337.70 |
| 25 | 241.10 | 361.70 |
| 26 | 257.30 | 386.00 |
| 27 | 273.30 | 410.00 |
| 28 | 289.30 | 434.00 |
| 29 | 305.40 | 458.10 |
| 30 | 321.40 | 482.10 |
June 1982
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $17.50 | $26.30 |
| 12 | 34.60 | 52.00 |
| 13 | 51.90 | 78.00 |
| 14 | 69.10 | 103.80 |
| 15 | 86.30 | 129.60 |
| 16 | 103.70 | 155.60 |
| 17 | 120.90 | 181.30 |
| 18 | 138.20 | 207.30 |
| 19 | 155.40 | 233.10 |
| 20 | 172.50 | 258.90 |
| 21 | 189.90 | 285.00 |
| 22 | 207.10 | 310.80 |
| 23 | 224.50 | 336.90 |
| 24 | 241.70 | 362.60 |
| 25 | 258.90 | 388.40 |
| 26 | 276.30 | 414.50 |
| 27 | 293.50 | 440.30 |
| 28 | 310.70 | 466.10 |
| 29 | 327.90 | 491.90 |
| 30 | 345.10 | 517.70 |
December 1983
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $18.10 | $27.20 |
| 12 | 35.80 | 53.80 |
| 13 | 53.70 | 80.70 |
| 14 | 71.50 | 107.40 |
| 15 | 89.30 | 134.10 |
| 16 | 107.30 | 161.00 |
| 17 | 125.10 | 187.60 |
| 18 | 143.00 | 214.50 |
| 19 | 160.80 | 241.20 |
| 20 | 178.50 | 267.90 |
| 21 | 196.50 | 294.90 |
| 22 | 214.30 | 321.60 |
| 23 | 232.30 | 348.60 |
| 24 | 250.10 | 375.20 |
| 25 | 267.90 | 401.90 |
| 26 | 285.90 | 429.00 |
| 27 | 303.70 | 455.70 |
| 28 | 321.50 | 482.40 |
| 29 | 339.30 | 509.10 |
| 30 | 357.10 | 535.80 |
December 1984
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $18.70 | $28.10 |
| 12 | 37.00 | 55.60 |
| 13 | 55.50 | 83.50 |
| 14 | 74.00 | 111.10 |
| 15 | 92.40 | 138.70 |
| 16 | 111.00 | 166.60 |
| 17 | 129.40 | 194.10 |
| 18 | 148.00 | 222.00 |
| 19 | 166.40 | 249.60 |
| 20 | 184.70 | 277.20 |
| 21 | 203.30 | 305.20 |
| 22 | 221.80 | 332.80 |
| 23 | 240.40 | 360.80 |
| 24 | 258.80 | 388.30 |
| 25 | 277.20 | 415.90 |
| 26 | 295.90 | 444.00 |
| 27 | 314.30 | 471.60 |
| 28 | 332.70 | 499.20 |
| 29 | 351.10 | 526.90 |
| 30 | 369.50 | 554.50 |
December 1985
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $19.20 | $28.90 |
| 12 | 38.10 | 57.30 |
| 13 | 57.20 | 86.00 |
| 14 | 76.20 | 114.50 |
| 15 | 95.20 | 142.90 |
| 16 | 114.40 | 171.70 |
| 17 | 133.40 | 200.10 |
| 18 | 152.50 | 228.80 |
| 19 | 171.50 | 257.30 |
| 20 | 190.40 | 285.70 |
| 21 | 209.60 | 314.60 |
| 22 | 228.60 | 343.10 |
| 23 | 247.80 | 371.90 |
| 24 | 266.80 | 400.30 |
| 25 | 285.70 | 428.70 |
| 26 | 305.00 | 457.70 |
| 27 | 324.00 | 486.20 |
| 28 | 343.00 | 514.60 |
| 29 | 361.90 | 543.20 |
| 30 | 380.90 | 571.60 |
December 1986
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $19.40 | $29.20 |
| 12 | 38.50 | 58.00 |
| 13 | 57.90 | 87.10 |
| 14 | 77.10 | 115.90 |
| 15 | 96.40 | 144.70 |
| 16 | 115.80 | 173.90 |
| 17 | 135.10 | 202.70 |
| 18 | 154.40 | 231.70 |
| 19 | 173.70 | 260.60 |
| 20 | 192.80 | 289.40 |
| 21 | 212.30 | 318.60 |
| 22 | 231.50 | 347.50 |
| 23 | 251.00 | 376.70 |
| 24 | 270.20 | 405.50 |
| 25 | 289.40 | 434.20 |
| 26 | 308.90 | 463.60 |
| 27 | 328.20 | 492.50 |
| 28 | 347.40 | 521.20 |
| 29 | 366.60 | 550.20 |
| 30 | 385.80 | 579.00 |
December 1987
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $20.20 | $30.40 |
| 12 | 40.10 | 60.40 |
| 13 | 60.30 | 90.70 |
| 14 | 80.30 | 120.70 |
| 15 | 100.40 | 150.70 |
| 16 | 120.60 | 181.20 |
| 17 | 140.70 | 211.20 |
| 18 | 160.80 | 241.40 |
| 19 | 180.90 | 271.50 |
| 20 | 200.80 | 301.50 |
| 21 | 221.20 | 331.90 |
| 22 | 241.20 | 362.00 |
| 23 | 261.50 | 392.50 |
| 24 | 281.50 | 422.50 |
| 25 | 301.50 | 452.40 |
| 26 | 321.80 | 483.00 |
| 27 | 341.90 | 513.10 |
| 28 | 361.90 | 543.00 |
| 29 | 381.90 | 573.30 |
| 30 | 402.00 | 603.30 |
December 1988
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $21.00 | $31.60 |
| 12 | 41.70 | 62.80 |
| 13 | 62.70 | 94.30 |
| 14 | 83.50 | 125.50 |
| 15 | 104.40 | 156.70 |
| 16 | 125.40 | 188.40 |
| 17 | 146.30 | 219.60 |
| 18 | 167.20 | 251.00 |
| 19 | 188.10 | 282.30 |
| 20 | 208.80 | 313.50 |
| 21 | 230.00 | 345.10 |
| 22 | 250.80 | 376.40 |
| 23 | 271.90 | 408.20 |
| 24 | 292.70 | 439.40 |
| 25 | 313.50 | 470.40 |
| 26 | 334.60 | 502.30 |
| 27 | 355.50 | 533.60 |
| 28 | 376.30 | 564.70 |
| 29 | 397.10 | 596.20 |
| 30 | 418.00 | 627.40 |
December 1989
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $21.90 | $33.00 |
| 12 | 43.60 | 65.70 |
| 13 | 65.60 | 98.70 |
| 14 | 87.40 | 131.30 |
| 15 | 109.30 | 164.00 |
| 16 | 131.20 | 197.20 |
| 17 | 153.10 | 229.90 |
| 18 | 175.00 | 262.70 |
| 19 | 196.90 | 295.50 |
| 20 | 218.60 | 328.20 |
| 21 | 240.80 | 361.30 |
| 22 | 262.50 | 394.00 |
| 23 | 284.60 | 427.30 |
| 24 | 306.40 | 460.00 |
| 25 | 328.20 | 492.50 |
| 26 | 350.30 | 525.90 |
| 27 | 372.20 | 558.60 |
| 28 | 393.90 | 591.20 |
| 29 | 415.70 | 624.20 |
| 30 | 437.60 | 656.80 |
December 1990
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $23.00 | $34.70 |
| 12 | 45.90 | 69.20 |
| 13 | 69.10 | 104.00 |
| 14 | 92.10 | 138.30 |
| 15 | 115.20 | 172.80 |
| 16 | 138.20 | 207.80 |
| 17 | 161.30 | 242.30 |
| 18 | 184.40 | 276.80 |
| 19 | 207.50 | 311.40 |
| 20 | 230.40 | 345.90 |
| 21 | 253.80 | 380.80 |
| 22 | 276.60 | 415.20 |
| 23 | 299.90 | 450.30 |
| 24 | 322.90 | 484.80 |
| 25 | 345.90 | 519.00 |
| 26 | 369.20 | 554.20 |
| 27 | 392.20 | 588.70 |
| 28 | 415.10 | 623.10 |
| 29 | 438.10 | 657.90 |
| 30 | 461.20 | 692.20 |
December 1991
| I. Years of coverage | II. Primary insurance amount | III. Maximum family benefit |
|---|---|---|
| 11 | $23.80 | $35.90 |
| 12 | 47.50 | 71.70 |
| 13 | 71.60 | 107.80 |
| 14 | 95.50 | 143.40 |
| 15 | 119.40 | 179.10 |
| 16 | 143.30 | 215.40 |
| 17 | 167.20 | 251.20 |
| 18 | 191.20 | 287.00 |
| 19 | 215.10 | 322.90 |
| 20 | 238.90 | 358.60 |
| 21 | 263.10 | 394.80 |
| 22 | 286.80 | 430.50 |
| 23 | 310.90 | 466.90 |
| 24 | 334.80 | 502.70 |
| 25 | 358.60 | 538.20 |
| 26 | 382.80 | 574.70 |
| 27 | 406.70 | 610.40 |
| 28 | 430.40 | 646.10 |
| 29 | 454.30 | 682.20 |
| 30 | 478.20 | 717.80 |
Note: The amounts shown in the above table for years of coverage less than 19 are not payable for June 1981 through December 1981 because the corresponding values shown in column II are less than the $135.70 minimum primary insurance amount payable for that period. For months after December 1981, a special minimum primary insurance amount of $128.70 will be payable.
Appendix VI to Subpart C of Part 404 - Percentage of Automatic Increases in Primary Insurance Amounts Since 1978
20:2.0.1.1.5.3.124.44.9 : Appendix VI
Appendix VI to Subpart C of Part 404 - Percentage of Automatic Increases in Primary Insurance Amounts Since 1978| Effective date | Percentage increase |
|---|---|
| 06/79 | 9.9 |
| 06/80 | 14.3 |
| 06/81 | 11.2 |
| 06/82 | 7.4 |
| 12/83 | 3.5 |
| 12/84 | 3.5 |
| 12/85 | 3.1 |
| 12/86 | 1.3 |
| 12/87 | 4.2 |
| 12/88 | 4.0 |
| 12/89 | 4.7 |
| 12/90 | 5.4 |
| 12/91 | 3.7 |
Appendix VII to Subpart C of Part 404 - “Old-Law” Contribution and Benefit Base
20:2.0.1.1.5.3.124.44.10 : Appendix VII
Appendix VII to Subpart C of Part 404 - “Old-Law” Contribution and Benefit BaseExplanation: We use these figures to determine the earnings needed for a year of coverage for years after 1978 (see § 404.261 and appendix IV). This is the contribution and benefit base that would have been effective under the Social Security Act without the enactment of the 1977 amendments.
| Year | Amount |
|---|---|
| 1979 | $18,900 |
| 1980 | 20,400 |
| 1981 | 22,200 |
| 1982 | 24,300 |
| 1983 | 26,700 |
| 1984 | 28,200 |
| 1985 | 29,700 |
| 1986 | 31,500 |
| 1987 | 32,700 |
| 1988 | 33,600 |
| 1989 | 35,700 |
| 1990 | 38,100 |
| 1991 | 39,600 |
| 1992 | 41,400 |
Appendix 1 to Subpart P of Part 404 - Listing of Impairments
20:2.0.1.1.5.16.200.105.11 :
Appendix 1 to Subpart P of Part 404 - Listing of Impairments Link to an amendment published at 85 FR 78175, Dec. 3, 2020.The body system listings in parts A and B of the Listing of Impairments will no longer be effective on the following dates unless extended by the Commissioner or revised and promulgated again.
1. Low Birth Weight and Failure to Thrive (100.00): August 12, 2022. 2. Musculoskeletal System (1.00 and 101.00): February 4, 2022. 3. Special Senses and Speech (2.00 and 102.00): June 3, 2022. 4. Respiratory Disorders (3.00 and 103.00): December 10, 2021 5. Cardiovascular System (4.00 and 104.00): February 4, 2022. 6. Digestive System (5.00 and 105.00): February 4, 2022. 7. Genitourinary Disorders (6.00 and 106.00): December 10, 2021. 8. Hematological Disorders (7.00 and 107.00): June 3, 2022. 9. Skin Disorders (8.00 and 108.00): February 4, 2022. 10. Endocrine Disorders (9.00 and 109.00): August 12, 2022. 11. Congenital Disorders That Affect Multiple Body Systems (10.00 and 110.00): June 3, 2022. 12. Neurological Disorders (11.00 and 111.00): September 29, 2021. 13. Mental Disorders (12.00 and 112.00): January 17, 2022. 14. Cancer (Malignant Neoplastic Diseases) (13.00 and 113.00): August 12, 2022. 15. Immune System Disorders (14.00 and 114.00): February 4, 2022. Part ACriteria applicable to individuals age 18 and over and to children under age 18 where criteria are appropriate.
Sec. 1.00 Musculoskeletal System. 2.00 Special Senses and Speech. 3.00 Respiratory Disorders. 4.00 Cardiovascular System. 5.00 Digestive System. 6.00 Genitourinary Disorders. 7.00 Hematological Disorders. 8.00 Skin Disorders. 9.00 Endocrine Disorders. 10.00 Congenital Disorders That Affect Multiple Body Systems 11.00 Neurological Disorders. 12.00 Mental Disorders. 13.00 Cancer (Malignant Neoplastic Diseases). 14.00 Immune System Disorders. 1.00 Musculoskeletal SystemA. Disorders of the musculoskeletal system may result from hereditary, congenital, or acquired pathologic processes. Impairments may result from infectious, inflammatory, or degenerative processes, traumatic or developmental events, or neoplastic, vascular, or toxic/metabolic diseases.
B. Loss of function.
1. General. Under this section, loss of function may be due to bone or joint deformity or destruction from any cause; miscellaneous disorders of the spine with or without radiculopathy or other neurological deficits; amputation; or fractures or soft tissue injuries, including burns, requiring prolonged periods of immobility or convalescence. The provisions of 1.02 and 1.03 notwithstanding, inflammatory arthritis is evaluated under 14.09 (see 14.00D6). Impairments with neurological causes are to be evaluated under 11.00ff.
2. How We Define Loss of Function in These Listingsa. General. Regardless of the cause(s) of a musculoskeletal impairment, functional loss for purposes of these listings is defined as the inability to ambulate effectively on a sustained basis for any reason, including pain associated with the underlying musculoskeletal impairment, or the inability to perform fine and gross movements effectively on a sustained basis for any reason, including pain associated with the underlying musculoskeletal impairment. The inability to ambulate effectively or the inability to perform fine and gross movements effectively must have lasted, or be expected to last, for at least 12 months. For the purposes of these criteria, consideration of the ability to perform these activities must be from a physical standpoint alone. When there is an inability to perform these activities due to a mental impairment, the criteria in 12.00ff are to be used. We will determine whether an individual can ambulate effectively or can perform fine and gross movements effectively based on the medical and other evidence in the case record, generally without developing additional evidence about the individual's ability to perform the specific activities listed as examples in 1.00B2b(2) and 1.00B2c.
b. What We Mean by Inability To Ambulate Effectively(1) Definition. Inability to ambulate effectively means an extreme limitation of the ability to walk; i.e., an impairment(s) that interferes very seriously with the individual's ability to independently initiate, sustain, or complete activities. Ineffective ambulation is defined generally as having insufficient lower extremity functioning (see 1.00J) to permit independent ambulation without the use of a hand-held assistive device(s) that limits the functioning of both upper extremities. (Listing 1.05C is an exception to this general definition because the individual has the use of only one upper extremity due to amputation of a hand.)
(2) To ambulate effectively, individuals must be capable of sustaining a reasonable walking pace over a sufficient distance to be able to carry out activities of daily living. They must have the ability to travel without companion assistance to and from a place of employment or school. Therefore, examples of ineffective ambulation include, but are not limited to, the inability to walk without the use of a walker, two crutches or two canes, the inability to walk a block at a reasonable pace on rough or uneven surfaces, the inability to use standard public transportation, the inability to carry out routine ambulatory activities, such as shopping and banking, and the inability to climb a few steps at a reasonable pace with the use of a single hand rail. The ability to walk independently about one's home without the use of assistive devices does not, in and of itself, constitute effective ambulation.
c. What we mean by inability to perform fine and gross movements effectively. Inability to perform fine and gross movements effectively means an extreme loss of function of both upper extremities; i.e., an impairment(s) that interferes very seriously with the individual's ability to independently initiate, sustain, or complete activities. To use their upper extremities effectively, individuals must be capable of sustaining such functions as reaching, pushing, pulling, grasping, and fingering to be able to carry out activities of daily living. Therefore, examples of inability to perform fine and gross movements effectively include, but are not limited to, the inability to prepare a simple meal and feed oneself, the inability to take care of personal hygiene, the inability to sort and handle papers or files, and the inability to place files in a file cabinet at or above waist level.
d. Pain or other symptoms. Pain or other symptoms may be an important factor contributing to functional loss. In order for pain or other symptoms to be found to affect an individual's ability to perform basic work activities, medical signs or laboratory findings must show the existence of a medically determinable impairment(s) that could reasonably be expected to produce the pain or other symptoms. The musculoskeletal listings that include pain or other symptoms among their criteria also include criteria for limitations in functioning as a result of the listed impairment, including limitations caused by pain. It is, therefore, important to evaluate the intensity and persistence of such pain or other symptoms carefully in order to determine their impact on the individual's functioning under these listings. See also §§ 404.1525(f) and 404.1529 of this part, and §§ 416.925(f) and 416.929 of part 416 of this chapter.
C. Diagnosis and Evaluation1. General. Diagnosis and evaluation of musculoskeletal impairments should be supported, as applicable, by detailed descriptions of the joints, including ranges of motion, condition of the musculature (e.g., weakness, atrophy), sensory or reflex changes, circulatory deficits, and laboratory findings, including findings on x-ray or other appropriate medically acceptable imaging. Medically acceptable imaging includes, but is not limited to, x-ray imaging, computerized axial tomography (CAT scan) or magnetic resonance imaging (MRI), with or without contrast material, myelography, and radionuclear bone scans. “Appropriate” means that the technique used is the proper one to support the evaluation and diagnosis of the impairment.
2. Purchase of certain medically acceptable imaging. While any appropriate medically acceptable imaging is useful in establishing the diagnosis of musculoskeletal impairments, some tests, such as CAT scans and MRIs, are quite expensive, and we will not routinely purchase them. Some, such as myelograms, are invasive and may involve significant risk. We will not order such tests. However, when the results of any of these tests are part of the existing evidence in the case record we will consider them together with the other relevant evidence.
3. Consideration of electrodiagnostic procedures. Electrodiagnostic procedures may be useful in establishing the clinical diagnosis, but do not constitute alternative criteria to the requirements of 1.04.
D. The physical examination must include a detailed description of the rheumatological, orthopedic, neurological, and other findings appropriate to the specific impairment being evaluated. These physical findings must be determined on the basis of objective observation during the examination and not simply a report of the individual's allegation; e.g., “He says his leg is weak, numb.” Alternative testing methods should be used to verify the abnormal findings; e.g., a seated straight-leg raising test in addition to a supine straight-leg raising test. Because abnormal physical findings may be intermittent, their presence over a period of time must be established by a record of ongoing management and evaluation. Care must be taken to ascertain that the reported examination findings are consistent with the individual's daily activities.
E. Examination of the Spine1. General. Examination of the spine should include a detailed description of gait, range of motion of the spine given quantitatively in degrees from the vertical position (zero degrees) or, for straight-leg raising from the sitting and supine position (zero degrees), any other appropriate tension signs, motor and sensory abnormalities, muscle spasm, when present, and deep tendon reflexes. Observations of the individual during the examination should be reported; e.g., how he or she gets on and off the examination table. Inability to walk on the heels or toes, to squat, or to arise from a squatting position, when appropriate, may be considered evidence of significant motor loss. However, a report of atrophy is not acceptable as evidence of significant motor loss without circumferential measurements of both thighs and lower legs, or both upper and lower arms, as appropriate, at a stated point above and below the knee or elbow given in inches or centimeters. Additionally, a report of atrophy should be accompanied by measurement of the strength of the muscle(s) in question generally based on a grading system of 0 to 5, with 0 being complete loss of strength and 5 being maximum strength. A specific description of atrophy of hand muscles is acceptable without measurements of atrophy but should include measurements of grip and pinch strength.
2. When neurological abnormalities persist. Neurological abnormalities may not completely subside after treatment or with the passage of time. Therefore, residual neurological abnormalities that persist after it has been determined clinically or by direct surgical or other observation that the ongoing or progressive condition is no longer present will not satisfy the required findings in 1.04. More serious neurological deficits (paraparesis, paraplegia) are to be evaluated under the criteria in 11.00ff.
F. Major joints refers to the major peripheral joints, which are the hip, knee, shoulder, elbow, wrist-hand, and ankle-foot, as opposed to other peripheral joints (e.g., the joints of the hand or forefoot) or axial joints (i.e., the joints of the spine.) The wrist and hand are considered together as one major joint, as are the ankle and foot. Since only the ankle joint, which consists of the juncture of the bones of the lower leg (tibia and fibula) with the hindfoot (tarsal bones), but not the forefoot, is crucial to weight bearing, the ankle and foot are considered separately in evaluating weight bearing.
G. Measurements of joint motion are based on the techniques described in the chapter on the extremities, spine, and pelvis in the current edition of the “Guides to the Evaluation of Permanent Impairment” published by the American Medical Association.
H. Documentation1. General. Musculoskeletal impairments frequently improve with time or respond to treatment. Therefore, a longitudinal clinical record is generally important for the assessment of severity and expected duration of an impairment unless the claim can be decided favorably on the basis of the current evidence.
2. Documentation of medically prescribed treatment and response. Many individuals, especially those who have listing-level impairments, will have received the benefit of medically prescribed treatment. Whenever evidence of such treatment is available it must be considered.
3. When there is no record of ongoing treatment. Some individuals will not have received ongoing treatment or have an ongoing relationship with the medical community despite the existence of a severe impairment(s). In such cases, evaluation will be made on the basis of the current objective medical evidence and other available evidence, taking into consideration the individual's medical history, symptoms, and medical source opinions. Even though an individual who does not receive treatment may not be able to show an impairment that meets the criteria of one of the musculoskeletal listings, the individual may have an impairment(s) equivalent in severity to one of the listed impairments or be disabled based on consideration of his or her residual functional capacity (RFC) and age, education and work experience.
4. Evaluation when the criteria of a musculoskeletal listing are not met. These listings are only examples of common musculoskeletal disorders that are severe enough to prevent a person from engaging in gainful activity. Therefore, in any case in which an individual has a medically determinable impairment that is not listed, an impairment that does not meet the requirements of a listing, or a combination of impairments no one of which meets the requirements of a listing, we will consider medical equivalence. (See §§ 404.1526 and 416.926.) Individuals who have an impairment(s) with a level of severity that does not meet or equal the criteria of the musculoskeletal listings may or may not have the RFC that would enable them to engage in substantial gainful activity. Evaluation of the impairment(s) of these individuals should proceed through the final steps of the sequential evaluation process in §§ 404.1520 and 416.920 (or, as appropriate, the steps in the medical improvement review standard in §§ 404.1594 and 416.994).
I. Effects of Treatment1. General. Treatments for musculoskeletal disorders may have beneficial effects or adverse side effects. Therefore, medical treatment (including surgical treatment) must be considered in terms of its effectiveness in ameliorating the signs, symptoms, and laboratory abnormalities of the disorder, and in terms of any side effects that may further limit the individual.
2. Response to treatment. Response to treatment and adverse consequences of treatment may vary widely. For example, a pain medication may relieve an individual's pain completely, partially, or not at all. It may also result in adverse effects, e.g., drowsiness, dizziness, or disorientation, that compromise the individual's ability to function. Therefore, each case must be considered on an individual basis, and include consideration of the effects of treatment on the individual's ability to function.
3. Documentation. A specific description of the drugs or treatment given (including surgery), dosage, frequency of administration, and a description of the complications or response to treatment should be obtained. The effects of treatment may be temporary or long-term. As such, the finding regarding the impact of treatment must be based on a sufficient period of treatment to permit proper consideration or judgment about future functioning.
J. Orthotic, Prosthetic, or Assistive Devices1. General. Consistent with clinical practice, individuals with musculoskeletal impairments may be examined with and without the use of any orthotic, prosthetic, or assistive devices as explained in this section.
2. Orthotic devices. Examination should be with the orthotic device in place and should include an evaluation of the individual's maximum ability to function effectively with the orthosis. It is unnecessary to routinely evaluate the individual's ability to function without the orthosis in place. If the individual has difficulty with, or is unable to use, the orthotic device, the medical basis for the difficulty should be documented. In such cases, if the impairment involves a lower extremity or extremities, the examination should include information on the individual's ability to ambulate effectively without the device in place unless contraindicated by the medical judgment of a physician who has treated or examined the individual.
3. Prosthetic devices. Examination should be with the prosthetic device in place. In amputations involving a lower extremity or extremities, it is unnecessary to evaluate the individual's ability to walk without the prosthesis in place. However, the individual's medical ability to use a prosthesis to ambulate effectively, as defined in 1.00B2b, should be evaluated. The condition of the stump should be evaluated without the prosthesis in place.
4. Hand-held assistive devices. When an individual with an impairment involving a lower extremity or extremities uses a hand-held assistive device, such as a cane, crutch or walker, examination should be with and without the use of the assistive device unless contraindicated by the medical judgment of a physician who has treated or examined the individual. The individual's ability to ambulate with and without the device provides information as to whether, or the extent to which, the individual is able to ambulate without assistance. The medical basis for the use of any assistive device (e.g., instability, weakness) should be documented. The requirement to use a hand-held assistive device may also impact on the individual's functional capacity by virtue of the fact that one or both upper extremities are not available for such activities as lifting, carrying, pushing, and pulling.
K. Disorders of the spine, listed in 1.04, result in limitations because of distortion of the bony and ligamentous architecture of the spine and associated impingement on nerve roots (including the cauda equina) or spinal cord. Such impingement on nerve tissue may result from a herniated nucleus pulposus, spinal stenosis, arachnoiditis, or other miscellaneous conditions.
1. Herniated nucleus pulposus is a disorder frequently associated with the impingement of a nerve root. Nerve root compression results in a specific neuro-anatomic distribution of symptoms and signs depending upon the nerve root(s) compromised.
2. Spinal Arachnoiditisa. General. Spinal arachnoiditis is a condition characterized by adhesive thickening of the arachnoid which may cause intermittent ill-defined burning pain and sensory dysesthesia, and may cause neurogenic bladder or bowel incontinence when the cauda equina is involved.
b. Documentation. Although the cause of spinal arachnoiditis is not always clear, it may be associated with chronic compression or irritation of nerve roots (including the cauda equina) or the spinal cord. For example, there may be evidence of spinal stenosis, or a history of spinal trauma or meningitis. Diagnosis must be confirmed at the time of surgery by gross description, microscopic examination of biopsied tissue, or by findings on appropriate medically acceptable imaging. Arachnoiditis is sometimes used as a diagnosis when such a diagnosis is unsupported by clinical or laboratory findings. Therefore, care must be taken to ensure that the diagnosis is documented as described in 1.04B. Individuals with arachnoiditis, particularly when it involves the lumbosacral spine, are generally unable to sustain any given position or posture for more than a short period of time due to pain.
3. Lumbar spinal stenosis is a condition that may occur in association with degenerative processes, or as a result of a congenital anomaly or trauma, or in association with Paget's disease of the bone. Pseudoclaudication, which may result from lumbar spinal stenosis, is manifested as pain and weakness, and may impair ambulation. Symptoms are usually bilateral, in the low back, buttocks, or thighs, although some individuals may experience only leg pain and, in a few cases, the leg pain may be unilateral. The pain generally does not follow a particular neuro-anatomical distribution, i.e., it is distinctly different from the radicular type of pain seen with a herniated intervertebral disc, is often of a dull, aching quality, which may be described as “discomfort” or an “unpleasant sensation,” or may be of even greater severity, usually in the low back and radiating into the buttocks region bilaterally. The pain is provoked by extension of the spine, as in walking or merely standing, but is reduced by leaning forward. The distance the individual has to walk before the pain comes on may vary. Pseudoclaudication differs from peripheral vascular claudication in several ways. Pedal pulses and Doppler examinations are unaffected by pseudoclaudication. Leg pain resulting from peripheral vascular claudication involves the calves, and the leg pain in vascular claudication is ordinarily more severe than any back pain that may also be present. An individual with vascular claudication will experience pain after walking the same distance time after time, and the pain will be relieved quickly when walking stops.
4. Other miscellaneous conditions that may cause weakness of the lower extremities, sensory changes, areflexia, trophic ulceration, bladder or bowel incontinence, and that should be evaluated under 1.04 include, but are not limited to, osteoarthritis, degenerative disc disease, facet arthritis, and vertebral fracture. Disorders such as spinal dysrhaphism (e.g., spina bifida), diastematomyelia, and tethered cord syndrome may also cause such abnormalities. In these cases, there may be gait difficulty and deformity of the lower extremities based on neurological abnormalities, and the neurological effects are to be evaluated under the criteria in 11.00ff.
L. Abnormal curvatures of the spine. Abnormal curvatures of the spine (specifically, scoliosis, kyphosis and kyphoscoliosis) can result in impaired ambulation, but may also adversely affect functioning in body systems other than the musculoskeletal system. For example, an individual's ability to breathe may be affected; there may be cardiac difficulties (e.g., impaired myocardial function); or there may be disfigurement resulting in withdrawal or isolation. When there is impaired ambulation, evaluation of equivalence may be made by reference to 14.09A. When the abnormal curvature of the spine results in symptoms related to fixation of the dorsolumbar or cervical spine, evaluation of equivalence may be made by reference to 14.09C. When there is respiratory or cardiac involvement or an associated mental disorder, evaluation may be made under 3.00ff, 4.00ff, or 12.00ff, as appropriate. Other consequences should be evaluated according to the listing for the affected body system.
M. Under continuing surgical management, as used in 1.07 and 1.08, refers to surgical procedures and any other associated treatments related to the efforts directed toward the salvage or restoration of functional use of the affected part. It may include such factors as post-surgical procedures, surgical complications, infections, or other medical complications, related illnesses, or related treatments that delay the individual's attainment of maximum benefit from therapy. When burns are not under continuing surgical management, see 8.00F.
N. After maximum benefit from therapy has been achieved in situations involving fractures of an upper extremity (1.07), or soft tissue injuries (1.08), i.e., there have been no significant changes in physical findings or on appropriate medically acceptable imaging for any 6-month period after the last definitive surgical procedure or other medical intervention, evaluation must be made on the basis of the demonstrable residuals, if any. A finding that 1.07 or 1.08 is met must be based on a consideration of the symptoms, signs, and laboratory findings associated with recent or anticipated surgical procedures and the resulting recuperative periods, including any related medical complications, such as infections, illnesses, and therapies which impede or delay the efforts toward restoration of function. Generally, when there has been no surgical or medical intervention for 6 months after the last definitive surgical procedure, it can be concluded that maximum therapeutic benefit has been reached. Evaluation at this point must be made on the basis of the demonstrable residual limitations, if any, considering the individual's impairment-related symptoms, signs, and laboratory findings, any residual symptoms, signs, and laboratory findings associated with such surgeries, complications, and recuperative periods, and other relevant evidence.
O. Major function of the face and head, for purposes of listing 1.08, relates to impact on any or all of the activities involving vision, hearing, speech, mastication, and the initiation of the digestive process.
P. When surgical procedures have been performed, documentation should include a copy of the operative notes and available pathology reports.
Q. Effects of obesity. Obesity is a medically determinable impairment that is often associated with disturbance of the musculoskeletal system, and disturbance of this system can be a major cause of disability in individuals with obesity. The combined effects of obesity with musculoskeletal impairments can be greater than the effects of each of the impairments considered separately. Therefore, when determining whether an individual with obesity has a listing-level impairment or combination of impairments, and when assessing a claim at other steps of the sequential evaluation process, including when assessing an individual's residual functional capacity, adjudicators must consider any additional and cumulative effects of obesity.
1.01 Category of Impairments, Musculoskeletal1.02 Major dysfunction of a joint(s) (due to any cause): Characterized by gross anatomical deformity (e.g., subluxation, contracture, bony or fibrous ankylosis, instability) and chronic joint pain and stiffness with signs of limitation of motion or other abnormal motion of the affected joint(s), and findings on appropriate medically acceptable imaging of joint space narrowing, bony destruction, or ankylosis of the affected joint(s). With:
A. Involvement of one major peripheral weight-bearing joint (i.e., hip, knee, or ankle), resulting in inability to ambulate effectively, as defined in 1.00B2b;
orB. Involvement of one major peripheral joint in each upper extremity (i.e., shoulder, elbow, or wrist-hand), resulting in inability to perform fine and gross movements effectively, as defined in 1.00B2c.
1.03 Reconstructive surgery or surgical arthrodesis of a major weight-bearing joint, with inability to ambulate effectively, as defined in 1.00B2b, and return to effective ambulation did not occur, or is not expected to occur, within 12 months of onset.
1.04 Disorders of the spine (e.g., herniated nucleus pulposus, spinal arachnoiditis, spinal stenosis, osteoarthritis, degenerative disc disease, facet arthritis, vertebral fracture), resulting in compromise of a nerve root (including the cauda equina) or the spinal cord. With:
A. Evidence of nerve root compression characterized by neuro-anatomic distribution of pain, limitation of motion of the spine, motor loss (atrophy with associated muscle weakness or muscle weakness) accompanied by sensory or reflex loss and, if there is involvement of the lower back, positive straight-leg raising test (sitting and supine);
orB. Spinal arachnoiditis, confirmed by an operative note or pathology report of tissue biopsy, or by appropriate medically acceptable imaging, manifested by severe burning or painful dysesthesia, resulting in the need for changes in position or posture more than once every 2 hours;
orC. Lumbar spinal stenosis resulting in pseudoclaudication, established by findings on appropriate medically acceptable imaging, manifested by chronic nonradicular pain and weakness, and resulting in inability to ambulate effectively, as defined in 1.00B2b.
1.05 Amputation (due to any cause).
A. Both hands; or
orB. One or both lower extremities at or above the tarsal region, with stump complications resulting in medical inability to use a prosthetic device to ambulate effectively, as defined in 1.00B2b, which have lasted or are expected to last for at least 12 months;
orC. One hand and one lower extremity at or above the tarsal region, with inability to ambulate effectively, as defined in 1.00B2b; OR
D. Hemipelvectomy or hip disarticulation.
1.06 Fracture of the femur, tibia, pelvis, or one or more of the tarsal bones. With:
A. Solid union not evident on appropriate medically acceptable imaging and not clinically solid;
andB. Inability to ambulate effectively, as defined in 1.00B2b, and return to effective ambulation did not occur or is not expected to occur within 12 months of onset.
1.07 Fracture of an upper extremity with nonunion of a fracture of the shaft of the humerus, radius, or ulna, under continuing surgical management, as defined in 1.00M, directed toward restoration of functional use of the extremity, and such function was not restored or expected to be restored within 12 months of onset.
1.08 Soft tissue injury (e.g., burns) of an upper or lower extremity, trunk, or face and head, under continuing surgical management, as defined in 1.00M, directed toward the salvage or restoration of major function, and such major function was not restored or expected to be restored within 12 months of onset. Major function of the face and head is described in 1.00O.
2.00 Special Senses and SpeechA. How do we evaluate visual disorders?
1. What are visual disorders? Visual disorders are abnormalities of the eye, the optic nerve, the optic tracts, or the brain that may cause a loss of visual acuity or visual fields. A loss of visual acuity limits your ability to distinguish detail, read, or do fine work. A loss of visual fields limits your ability to perceive visual stimuli in the peripheral extent of vision.
2. How do we define statutory blindness? Statutory blindness is blindness as defined in sections 216(i)(1) and 1614(a)(2) of the Social Security Act (Act).
a. The Act defines blindness as central visual acuity of 20/200 or less in the better eye with the use of a correcting lens. We use your best-corrected central visual acuity for distance in the better eye when we determine if this definition is met. (For visual acuity testing requirements, see 2.00A5.)
b. The Act also provides that an eye that has a visual field limitation such that the widest diameter of the visual field subtends an angle no greater than 20 degrees is considered as having a central visual acuity of 20/200 or less. (For visual field testing requirements, see 2.00A6.)
c. You have statutory blindness only if your visual disorder meets the criteria of 2.02 or 2.03A. You do not have statutory blindness if your visual disorder medically equals the criteria of 2.02 or 2.03A or meets or medically equals the criteria of 2.03B, 2.03C, 2.04A, or 2.04B because your disability is based on criteria other than those in the statutory definition of blindness.
3. What evidence do we need to establish statutory blindness under title XVI? To establish that you have statutory blindness under title XVI, we need evidence showing only that your central visual acuity in your better eye or your visual field in your better eye meets the criteria in 2.00A2, provided that those measurements are consistent with the other evidence in your case record. We do not need documentation of the cause of your blindness. Also, there is no duration requirement for statutory blindness under title XVI (see §§ 416.981 and 416.983 of this chapter).
4. What evidence do we need to evaluate visual disorders, including those that result in statutory blindness under title II? To evaluate your visual disorder, we usually need a report of an eye examination that includes measurements of your best-corrected central visual acuity (see 2.00A5) or the extent of your visual fields (see 2.00A6), as appropriate. If you have visual acuity or visual field loss, we need documentation of the cause of the loss. A standard eye examination will usually indicate the cause of any visual acuity loss. A standard eye examination can also indicate the cause of some types of visual field deficits. Some disorders, such as cortical visual disorders, may result in abnormalities that do not appear on a standard eye examination. If the standard eye examination does not indicate the cause of your vision loss, we will request the information used to establish the presence of your visual disorder. If your visual disorder does not satisfy the criteria in 2.02, 2.03, or 2.04, we will request a description of how your visual disorder affects your ability to function.
5. How do we measure your best-corrected central visual acuity?
a. Visual acuity testing. When we need to measure your best-corrected central visual acuity (your optimal visual acuity attainable with the use of a corrective lens), we use visual acuity testing for distance that was carried out using Snellen methodology or any other testing methodology that is comparable to Snellen methodology.
(i) Your best-corrected central visual acuity for distance is usually measured by determining what you can see from 20 feet. If your visual acuity is measured for a distance other than 20 feet, we will convert it to a 20-foot measurement. For example, if your visual acuity is measured at 10 feet and is reported as 10/40, we will convert this measurement to 20/80.
(ii) A visual acuity recorded as CF (counts fingers), HM (hand motion only), LP or LPO (light perception or light perception only), or NLP (no light perception) indicates that no optical correction will improve your visual acuity. If your central visual acuity in an eye is recorded as CF, HM, LP or LPO, or NLP, we will determine that your best-corrected central visual acuity is 20/200 or less in that eye.
(iii) We will not use the results of pinhole testing or automated refraction acuity to determine your best-corrected central visual acuity. These tests provide an estimate of potential visual acuity but not an actual measurement of your best-corrected central visual acuity.
b. Other test charts. Most test charts that use Snellen methodology do not have lines that measure visual acuity between 20/100 and 20/200. Some test charts, such as the Bailey-Lovie or the Early Treatment Diabetic Retinopathy Study (ETDRS), used mostly in research settings, have such lines. If your visual acuity is measured with one of these charts, and you cannot read any of the letters on the 20/100 line, we will determine that you have statutory blindness based on a visual acuity of 20/200 or less. For example, if your best-corrected central visual acuity for distance in the better eye is 20/160 using an ETDRS chart, we will find that you have statutory blindness. Regardless of the type of test chart used, you do not have statutory blindness if you can read at least one letter on the 20/100 line. For example, if your best-corrected central visual acuity for distance in the better eye is 20/125 + 1 using an ETDRS chart, we will find that you do not have statutory blindness because you are able to read one letter on the 20/100 line.
c. Testing using a specialized lens. In some instances, you may have visual acuity testing performed using specialized lens, such as a contact lens. We will use the visual acuity measurements obtained with a specialized lens only if you have demonstrated the ability to use the specialized lens on a sustained basis. We will not use visual acuity measurements obtained with telescopic lenses.
d. Cycloplegic refraction is an examination of the eye performed after administering cycloplegic eye drops capable of relaxing the ability of the pupil to become smaller and temporarily paralyzing the focusing muscles. If your case record contains the results of cycloplegic refraction, we may use the results to determine your best-corrected central visual acuity. We will not purchase cycloplegic refraction.
e. Visual evoked response (VER) testing measures your response to visual events and can often detect dysfunction that is undetectable through other types of examinations. If you have an absent response to VER testing in your better eye, we will determine that your best-corrected central visual acuity is 20/200 or less in that eye and that your visual acuity loss satisfies the criterion in 2.02 when these test results are consistent with the other evidence in your case record. If you have a positive response to VER testing in an eye, we will not use that result to determine your best-corrected central visual acuity in that eye.
6. How do we measure your visual fields?
a. General. We generally need visual field testing when you have a visual disorder that could result in visual field loss, such as glaucoma, retinitis pigmentosa, or optic neuropathy, or when you display behaviors that suggest a visual field loss. When we need to measure the extent of your visual field loss, we use visual field testing (also referred to as perimetry) carried out using automated static threshold perimetry performed on an acceptable perimeter. (For perimeter requirements, see 2.00A9.)
b. Automated static threshold perimetry requirements.
(i) The test must use a white size III Goldmann stimulus and a 31.5 apostilb (asb) white background (or a 10 candela per square meter (cd/m 2) white background). The stimuli test locations must be no more than 6 degrees apart horizontally or vertically. Measurements must be reported on standard charts and include a description of the size and intensity of the test stimulus.
(ii) We measure the extent of your visual field loss by determining the portion of the visual field in which you can see a white III4e stimulus. The “III” refers to the standard Goldmann test stimulus size III (4 mm 2), and the “4e” refers to the standard Goldmann intensity filter (0 decibel (dB) attenuation, which allows presentation of the maximum luminance) used to determine the intensity of the stimulus.
(iii) In automated static threshold perimetry, the intensity of the stimulus varies. The intensity of the stimulus is expressed in decibels (dB). A perimeter's maximum stimulus luminance is usually assigned the value 0 dB. We need to determine the dB level that corresponds to a 4e intensity for the particular perimeter being used. We will then use the dB printout to determine which points you see at a 4e intensity level (a “seeing point”). For example:
A. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 10,000 asb, a 10 dB stimulus is equivalent to a 4e stimulus. Any point you see at 10 dB or greater is a seeing point.
B. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 4,000 asb, a 6 dB stimulus is equivalent to a 4e stimulus. Any point you see at 6 dB or greater is a seeing point.
C. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 1,000 asb, a 0 dB stimulus is equivalent to a 4e stimulus. Any point you see at 0 dB or greater is a seeing point.
c. Evaluation under 2.03A. To determine statutory blindness based on visual field loss in your better eye (2.03A), we need the results of a visual field test that measures the central 24 to 30 degrees of your visual field; that is, the area measuring 24 to 30 degrees from the point of fixation. Acceptable tests include the Humphrey Field Analyzer (HFA) 30-2, HFA 24-2, and Octopus 32.
d. Evaluation under 2.03B. To determine whether your visual field loss meets listing 2.03B, we use the mean deviation or defect (MD) from acceptable automated static threshold perimetry that measures the central 30 degrees of the visual field. MD is the average sensitivity deviation from normal values for all measured visual field locations. When using results from HFA tests, which report the MD as a negative number, we use the absolute value of the MD to determine whether your visual field loss meets listing 2.03B. We cannot use tests that do not measure the central 30 degrees of the visual field, such as the HFA 24-2, to determine if your impairment meets or medically equals 2.03B.
e. Other types of perimetry. If the evidence in your case contains visual field measurements obtained using manual or automated kinetic perimetry, such as Goldmann perimetry or the HFA “SSA Test Kinetic,” we can generally use these results if the kinetic test was performed using a white III4e stimulus projected on a white 31.5 asb (10 cd/m 2) background. Automated kinetic perimetry, such as the HFA “SSA Test Kinetic,” does not detect limitations in the central visual field because testing along a meridian stops when you see the stimulus. If your visual disorder has progressed to the point at which it is likely to result in a significant limitation in the central visual field, such as a scotoma (see 2.00A6h), we will not use automated kinetic perimetry to determine the extent of your visual field loss. Instead, we will determine the extent of your visual field loss using automated static threshold perimetry or manual kinetic perimetry.
f. Screening tests. We will not use the results of visual field screening tests, such as confrontation tests, tangent screen tests, or automated static screening tests, to determine that your impairment meets or medically equals a listing or to evaluate your residual functional capacity. We can consider normal results from visual field screening tests to determine whether your visual disorder is severe when these test results are consistent with the other evidence in your case record. (See §§ 404.1520(c), 404.1521, 416.920(c), and 416.921 of this chapter.) We will not consider normal test results to be consistent with the other evidence if the clinical findings indicate that your visual disorder has progressed to the point that it is likely to cause visual field loss, or you have a history of an operative procedure for retinal detachment.
g. Use of corrective lenses. You must not wear eyeglasses during visual field testing because they limit your field of vision. You may wear contact lenses to correct your visual acuity during the visual field test to obtain the most accurate visual field measurements. For this single purpose, you do not need to demonstrate that you have the ability to use the contact lenses on a sustained basis.
h. Scotoma. A scotoma is a field defect or non-seeing area (also referred to as a “blind spot”) in the visual field surrounded by a normal field or seeing area. When we measure your visual field, we subtract the length of any scotoma, other than the normal blind spot, from the overall length of any diameter on which it falls.
7. How do we determine your visual acuity efficiency, visual field efficiency, and visual efficiency?
a. General. Visual efficiency, a calculated value of your remaining visual function, is the combination of your visual acuity efficiency and your visual field efficiency expressed as a percentage.
b. Visual acuity efficiency. Visual acuity efficiency is a percentage that corresponds to the best-corrected central visual acuity for distance in your better eye. See Table 1.
Table 1 - Visual Acuity Efficiency
| Snellen best-corrected central visual acuity for distance | Visual acuity
efficiency (%) (2.04A) |
|
|---|---|---|
| English | Metric | |
| 20/16 | 6/5 | 100 |
| 20/20 | 6/6 | 100 |
| 20/25 | 6/7.5 | 95 |
| 20/30 | 6/9 | 90 |
| 20/40 | 6/12 | 85 |
| 20/50 | 6/15 | 75 |
| 20/60 | 6/18 | 70 |
| 20/70 | 6/21 | 65 |
| 20/80 | 6/24 | 60 |
| 20/100 | 6/30 | 50 |
c. Visual field efficiency. Visual field efficiency is a percentage that corresponds to the visual field in your better eye. Under 2.03C, we require kinetic perimetry to determine your visual field efficiency percentage. We calculate the visual field efficiency percentage by adding the number of degrees you see along the eight principal meridians found on a visual field chart (0, 45, 90, 135, 180, 225, 270, and 315) in your better eye and dividing by 5. For example, in Figure 1:
A. The diagram of the left eye illustrates a visual field, as measured with a III4e stimulus, contracted to 30 degrees in two meridians (180 and 225) and to 20 degrees in the remaining six meridians. The visual efficiency percentage of this field is: ((2 × 30) + (6 × 20)) ÷ 5 = 36 percent.
B. The diagram of the right eye illustrates the extent of a normal visual field as measured with a III4e stimulus. The sum of the eight principal meridians of this field is 500 degrees. The visual efficiency percentage of this field is 500 ÷ 5 = 100 percent.
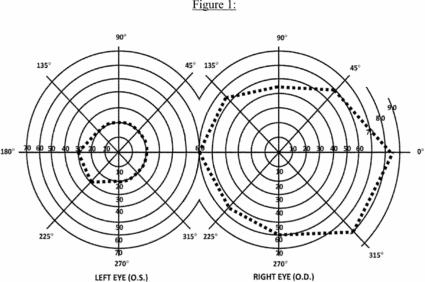
d. Visual efficiency. Under 2.04A, we calculate the visual efficiency percentage by multiplying your visual acuity efficiency percentage (see 2.00A7b) by your visual field efficiency percentage (see 2.00A7c) and dividing by 100. For example, if your visual acuity efficiency percentage is 75 and your visual field efficiency percentage is 36, your visual efficiency percentage is: (75 × 36) ÷ 100 = 27 percent.
8. How do we determine your visual acuity impairment value, visual field impairment value, and visual impairment value?
a. General. Visual impairment value, a calculated value of your loss of visual function, is the combination of your visual acuity impairment value and your visual field impairment value.
b. Visual acuity impairment value. Your visual acuity impairment value corresponds to the best-corrected central visual acuity for distance in your better eye. See Table 2.
Table 2 - Visual Acuity Impairment Value
| Snellen best-corrected central visual acuity for distance | Visual acuity impairment value (2.04B) |
|
| English | Metric | |
| 20/16 | 6/5 | 0.00 |
| 20/20 | 6/6 | 0.00 |
| 20/25 | 6/7.5 | 0.10 |
| 20/30 | 6/9 | 0.18 |
| 20/40 | 6/12 | 0.30 |
| 20/50 | 6/15 | 0.40 |
| 20/60 | 6/18 | 0.48 |
| 20/70 | 6/21 | 0.54 |
| 20/80 | 6/24 | 0.60 |
| 20/100 | 6/30 | 0.70 |
c. Visual field impairment value. Your visual field impairment value corresponds to the visual field in your better eye. Using the MD from acceptable automated static threshold perimetry, we calculate the visual field impairment value by dividing the absolute value of the MD by 22. For example, if your MD on an HFA 30-2 is −16, your visual field impairment value is: −16| ÷ 22 = 0.73.
d. Visual impairment value. Under 2.04B, we calculate the visual impairment value by adding your visual acuity impairment value (see 2.00A8b) and your visual field impairment value (see 2.00A8c). For example, if your visual acuity impairment value is 0.48 and your visual field impairment value is 0.73, your visual impairment value is: 0.48 + 0.73 = 1.21.
9. What are our requirements for an acceptable perimeter? We will use results from automated static threshold perimetry performed on a perimeter that:
a. Uses optical projection to generate the test stimuli.
b. Has an internal normative database for automatically comparing your performance with that of the general population.
c. Has a statistical analysis package that is able to calculate visual field indices, particularly MD.
d. Demonstrates the ability to correctly detect visual field loss and correctly identify normal visual fields.
e. Demonstrates good test-retest reliability.
f. Has undergone clinical validation studies by three or more independent laboratories with results published in peer-reviewed ophthalmic journals.
B. How do we evaluate hearing loss?
1. What evidence do we need?
a. We need evidence showing that you have a medically determinable impairment that causes your hearing loss and audiometric measurements of the severity of your hearing loss. We generally require both an otologic examination and audiometric testing to establish that you have a medically determinable impairment that causes your hearing loss. You should have this audiometric testing within 2 months of the otologic examination. Once we have evidence that you have a medically determinable impairment, we can use the results of later audiometric testing to assess the severity of your hearing loss without another otologic examination. We will consider your test scores together with any other relevant information we have about your hearing, including information from outside of the test setting.
b. The otologic examination must be performed by a licensed physician (medical or osteopathic doctor) or audiologist. It must include your medical history, your description of how your hearing loss affects you, and the physician's or audiologist's description of the appearance of the external ears (pinnae and external ear canals), evaluation of the tympanic membranes, and assessment of any middle ear abnormalities.
c. Audiometric testing must be performed by, or under the direct supervision of, a licensed audiologist or an otolaryngologist.
2. What audiometric testing do we need when you do not have a cochlear implant?
a. We generally need pure tone air conduction and bone conduction testing, speech reception threshold (SRT) testing (also referred to as “spondee threshold” or “ST” testing), and word recognition testing (also referred to as “word discrimination” or “speech discrimination” testing). This testing must be conducted in a sound-treated booth or room and must be in accordance with the most recently published standards of the American National Standards Institute (ANSI). Each ear must be tested separately.
b. You must not wear hearing aids during the testing. Additionally, a person described in 2.00B1c must perform an otoscopic examination immediately before the audiometric testing. (An otoscopic examination provides a description of the appearance of your external ear canals and an evaluation of the tympanic membranes. In these rules, we use the term to include otoscopic examinations performed by physicians and otoscopic inspections performed by audiologists and others.) The otoscopic examination must show that there are no conditions that would prevent valid audiometric testing, such as fluid in the ear, ear infection, or obstruction in an ear canal. The person performing the test should also report on any other factors, such as your cooperation with the test, that can affect the interpretation of the test results.
c. To determine whether your hearing loss meets the air and bone conduction criteria in 2.10A, we will average your air and bone conduction hearing thresholds at 500, 1000, and 2000 Hertz (Hz). If you do not have a response at a particular frequency, we will use a threshold of 5 decibels (dB) over the limit of the audiometer.
d. The SRT is the minimum dB level required for you to recognize 50 percent of the words on a standard list of spondee words. (Spondee words are two-syllable words that have equal stress on each syllable.) The SRT is usually within 10 dB of the average pure tone air conduction hearing thresholds at 500, 1000, and 2000 Hz. If the SRT is not within 10 dB of the average pure tone air conduction threshold, the reason for the discrepancy must be documented. If we cannot determine that there is a medical basis for the discrepancy, we will not use the results of the testing to determine whether your hearing loss meets a listing.
e. Word recognition testing determines your ability to recognize a standardized list of phonetically balanced monosyllabic words in the absence of any visual cues. This testing must be performed in quiet. The list may be recorded or presented live, but in either case the words should be presented at a level of amplification that will measure your maximum ability to discriminate words, usually 35 to 40 dB above your SRT. However, the amplification level used in the testing must be medically appropriate, and you must be able to tolerate it. If you cannot be tested at 35 to 40 dB above your SRT, the person who performs the test should report your word recognition testing score at your highest comfortable level of amplification.
3. What audiometric testing do we need when you have a cochlear implant?
a. If you have a cochlear implant, we will consider you to be disabled until 1 year after initial implantation.
b. After that period, we need word recognition testing performed with any version of the Hearing in Noise Test (HINT) to determine whether your impairment meets 2.11B. This testing must be conducted in quiet in a sound field. Your implant must be functioning properly and adjusted to your normal settings. The sentences should be presented at 60 dB HL (Hearing Level) and without any visual cues.
4. How do we evaluate your word recognition ability if you are not fluent in English?
If you are not fluent in English, you should have word recognition testing using an appropriate word list for the language in which you are most fluent. The person conducting the test should be fluent in the language used for the test. If there is no appropriate word list or no person who is fluent in the language and qualified to perform the test, it may not be possible to measure your word recognition ability. If your word recognition ability cannot be measured, your hearing loss cannot meet 2.10B or 2.11B. Instead, we will consider the facts of your case to determine whether you have difficulty understanding words in the language in which you are most fluent, and if so, whether that degree of difficulty medically equals 2.10B or 2.11B. For example, we will consider how you interact with family members, interpreters, and other persons who speak the language in which you are most fluent.
C. How do we evaluate vertigo associated with disturbances of labyrinthine-vestibular function, including Meniere's disease?
1. These disturbances of balance are characterized by an hallucination of motion or loss of position sense and a sensation of dizziness which may be constant or may occur in paroxysmal attacks. Nausea, vomiting, ataxia, and incapacitation are frequently observed, particularly during the acute attack. It is important to differentiate the report of rotary vertigo from that of “dizziness” which is described as lightheadedness, unsteadiness, confusion, or syncope.
2. Meniere's disease is characterized by paroxysmal attacks of vertigo, tinnitus, and fluctuating hearing loss. Remissions are unpredictable and irregular, but may be longlasting; hence, the severity of impairment is best determined after prolonged observation and serial reexaminations.
3. The diagnosis of a vestibular disorder requires a comprehensive neuro-otolaryngologic examination with a detailed description of the vertiginous episodes, including notation of frequency, severity, and duration of the attacks. Pure tone and speech audiometry with the appropriate special examinations, such as Bekesy audiometry, are necessary. Vestibular functions is assessed by positional and caloric testing, preferably by electronystagmography. When polytomograms, contrast radiography, or other special tests have been performed, copies of the reports of these tests should be obtained in addition to appropriate medically acceptable imaging reports of the skull and temporal bone. Medically acceptable imaging includes, but is not limited to, x-ray imaging, computerized axial tomography (CAT scan) or magnetic resonance imaging (MRI), with or without contrast material, myelography, and radionuclear bone scans. “Appropriate” means that the technique used is the proper one to support the evaluation and diagnosis of the impairment.
D. Loss of speech. In evaluating the loss of speech, the ability to produce speech by any means includes the use of mechanical or electronic devices that improve voice or articulation. Impairments of speech may also be evaluated under the body system for the underlying disorder, such as neurological disorders, 11.00ff.
E. How do we evaluate impairments that do not meet one of the special senses and speech listings?
1. These listings are only examples of common special senses and speech disorders that we consider severe enough to prevent an individual from doing any gainful activity. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
2. If you have a medically determinable impairment(s) that does not meet a listing, we will determine whether the impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926.) If you have an impairment(s) that does not meet or medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. Therefore, we proceed to the fourth, and if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920. When we decide whether you continue to be disabled, we use the rules in §§ 404.1594, 416.994, or 416.994a, as appropriate.
2.01 Category of Impairments, Special Senses and Speech
2.02 Loss of central visual acuity. Remaining vision in the better eye after best correction is 20/200 or less.
2.03 Contraction of the visual field in the better eye, with:
A. The widest diameter subtending an angle around the point of fixation no greater than 20 degrees.
ORB. An MD of 22 decibels or greater, determined by automated static threshold perimetry that measures the central 30 degrees of the visual field (see 2.00A6d).
ORC. A visual field efficiency of 20 percent or less, determined by kinetic perimetry (see 2.00A7c).
2.04 Loss of visual efficiency, or visual impairment, in the better eye:
A. A visual efficiency percentage of 20 or less after best correction (see 2.00A7d).
ORB. A visual impairment value of 1.00 or greater after best correction (see 2.00A8d).
2.07 Disturbance of labyrinthine-vestibular function (including Meniere's disease), characterized by a history of frequent attacks of balance disturbance, tinnitus, and progressive loss of hearing. With both A and B:
A. Disturbed function of vestibular labyrinth demonstrated by caloric or other vestibular tests; and
B. Hearing loss established by audiometry.
2.09 Loss of speech due to any cause, with inability to produce by any means speech that can be heard, understood, or sustained.
Table 1 - Percentage of Visual Acuity Efficiency Corresponding to the Best-Corrected Visual Acuity Measurement for Distance in the Better Eye
| Snellen | Percent visual acuity efficiency |
|
|---|---|---|
| English | Metric | |
| 20/16 | 6/5 | 100 |
| 20/20 | 6/6 | 100 |
| 20/25 | 6/7.5 | 95 |
| 20/30 | 6/9 | 90 |
| 20/40 | 6/12 | 85 |
| 20/50 | 6/15 | 75 |
| 20/60 | 6/18 | 70 |
| 20/70 | 6/21 | 65 |
| 20/80 | 6/24 | 60 |
| 20/100 | 6/30 | 50 |
Table 2 - Chart of Visual Fields
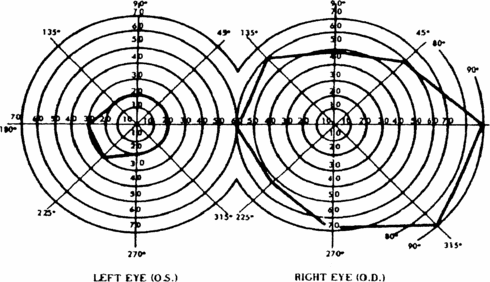
1. The diagram of the right eye illustrates the extent of a normal visual field as measured with a III4e stimulus. The sum of the eight principal meridians of this field is 500 degrees.
2. The diagram of the left eye illustrates a visual field contracted to 30 degrees in two meridians and to 20 degrees in the remaining six meridians. The percent of visual field efficiency of this field is: (2 × 30) + (6 × 20) = 180 ÷ 500 = 0.36 or 36 percent visual field efficiency.
2.10 Hearing loss not treated with cochlear implantation.
A. An average air conduction hearing threshold of 90 decibels or greater in the better ear and an average bone conduction hearing threshold of 60 decibels or greater in the better ear (see 2.00B2c).
OR
B. A word recognition score of 40 percent or less in the better ear determined using a standardized list of phonetically balanced monosyllabic words (see 2.00B2e).
2.11 Hearing loss treated with cochlear implantation.
A. Consider under a disability for 1 year after initial implantation.
OR
B. If more than 1 year after initial implantation, a word recognition score of 60 percent or less determined using the HINT (see 2.00B3b).
3.00 Respiratory DisordersA. Which disorders do we evaluate in this body system?
1. We evaluate respiratory disorders that result in obstruction (difficulty moving air out of the lungs) or restriction (difficulty moving air into the lungs), or that interfere with diffusion (gas exchange) across cell membranes in the lungs. Examples of such disorders and the listings we use to evaluate them include chronic obstructive pulmonary disease (chronic bronchitis and emphysema, 3.02), pulmonary fibrosis and pneumoconiosis (3.02), asthma (3.02 or 3.03), cystic fibrosis (3.04), and bronchiectasis (3.02 or 3.07). We also use listings in this body system to evaluate respiratory failure (3.04D or 3.14), chronic pulmonary hypertension (3.09), and lung transplantation (3.11).
2. We evaluate cancers affecting the respiratory system under the listings in 13.00. We evaluate the pulmonary effects of neuromuscular and autoimmune disorders under these listings or under the listings in 11.00 or 14.00, respectively.
B. What are the symptoms and signs of respiratory disorders? Symptoms and signs of respiratory disorders include dyspnea (shortness of breath), chest pain, coughing, wheezing, sputum production, hemoptysis (coughing up blood from the respiratory tract), use of accessory muscles of respiration, and tachypnea (rapid rate of breathing).
C. What abbreviations do we use in this body system?
1. ABG means arterial blood gas.
2. BiPAP means bi-level positive airway pressure ventilation.
3. BTPS means body temperature and ambient pressure, saturated with water vapor.
4. CF means cystic fibrosis.
5. CFRD means CF-related diabetes.
6. CFTR means CF transmembrane conductance regulator.
7. CO means carbon monoxide.
8. COPD means chronic obstructive pulmonary disease.
9. DLCO means diffusing capacity of the lungs for carbon monoxide.
10. FEV1 means forced expiratory volume in the first second of a forced expiratory maneuver.
11. FVC means forced vital capacity.
12. L means liter.
13. mL CO (STPD)/min/mmHg means milliliters of carbon monoxide at standard temperature and pressure, dry, per minute, per millimeter of mercury.
14. PaO2 means arterial blood partial pressure of oxygen.
15. PaCO2 means arterial blood partial pressure of carbon dioxide.
16. SpO2 means percentage of oxygen saturation of blood hemoglobin measured by pulse oximetry.
17. 6MWT means 6-minute walk test.
18. VI means volume of inhaled gas during a DLCO test.
D. What documentation do we need to evaluate your respiratory disorder?
1. We need medical evidence to document and assess the severity of your respiratory disorder. Medical evidence should include your medical history, physical examination findings, the results of imaging (see 3.00D3), pulmonary function tests (see 3.00D4), other relevant laboratory tests, and descriptions of any prescribed treatment and your response to it. We may not need all of this evidence depending on your particular respiratory disorder and its effects on you.
2. If you use supplemental oxygen, we still need medical evidence to establish the severity of your respiratory disorder.
3. Imaging refers to medical imaging techniques, such as x-ray and computerized tomography. The imaging must be consistent with the prevailing state of medical knowledge and clinical practice as the proper technique to support the evaluation of the disorder.
4. Pulmonary function tests include spirometry (which measures ventilation of the lungs), DLCO tests (which measure gas diffusion in the lungs), ABG tests (which measure the partial pressure of oxygen, PaO2, and carbon dioxide, PaCO2, in the arterial blood), and pulse oximetry (which measures oxygen saturation, SpO2, of peripheral blood hemoglobin).
E. What is spirometry and what are our requirements for an acceptable test and report?
1. Spirometry, which measures how well you move air into and out of your lungs, involves at least three forced expiratory maneuvers during the same test session. A forced expiratory maneuver is a maximum inhalation followed by a forced maximum exhalation, and measures exhaled volumes of air over time. The volume of air you exhale in the first second of the forced expiratory maneuver is the FEV1. The total volume of air that you exhale during the entire forced expiratory maneuver is the FVC. We use your highest FEV1 value to evaluate your respiratory disorder under 3.02A, 3.03A, and 3.04A, and your highest FVC value to evaluate your respiratory disorder under 3.02B, regardless of whether the values are from the same forced expiratory maneuver or different forced expiratory maneuvers.
2. We have the following requirements for spirometry under these listings:
a. You must be medically stable at the time of the test. Examples of when we would not consider you to be medically stable include when you are:
(i) Within 2 weeks of a change in your prescribed respiratory medication.
(ii) Experiencing, or within 30 days of completion of treatment for, a lower respiratory tract infection.
(iii) Experiencing, or within 30 days of completion of treatment for, an acute exacerbation (temporary worsening) of a chronic respiratory disorder. Wheezing by itself does not indicate that you are not medically stable.
(iv) Hospitalized, or within 30 days of a hospital discharge, for an acute myocardial infarction (heart attack).
b. During testing, if your FEV1 is less than 70 percent of your predicted normal value, we require repeat spirometry after inhalation of a bronchodilator to evaluate your respiratory disorder under these listings, unless it is medically contraindicated. If you used a bronchodilator before the test and your FEV1 is less than 70 percent of your predicted normal value, we still require repeat spirometry after inhalation of a bronchodilator unless the supervising physician determines that it is not safe for you to take a bronchodilator again (in which case we may need to reschedule the test). If you do not have post-bronchodilator spirometry, the test report must explain why. We can use the results of spirometry administered without bronchodilators when the use of bronchodilators is medically contraindicated.
c. Your forced expiratory maneuvers must be satisfactory. We consider a forced expiratory maneuver to be satisfactory when you exhale with maximum effort following a full inspiration, and when the test tracing has a sharp takeoff and rapid rise to peak flow, has a smooth contour, and either lasts for at least 6 seconds or maintains a plateau for at least 1 second.
3. The spirometry report must include the following information:
a. The date of the test and your name, age or date of birth, gender, and height without shoes. (We will assume that your recorded height on the date of the test is without shoes, unless we have evidence to the contrary.) If your spine is abnormally curved (for example, you have kyphoscoliosis), we will substitute the longest distance between your outstretched fingertips with your arms abducted 90 degrees in place of your height when this measurement is greater than your standing height without shoes.
b. Any factors, if applicable, that can affect the interpretation of the test results (for example, your cooperation or effort in doing the test).
c. Legible tracings of your forced expiratory maneuvers in a volume-time format showing your name and the date of the test for each maneuver.
4. If we purchase spirometry, the medical source we designate to administer the test is solely responsible for deciding whether it is safe for you to do the test and for how to administer it.
F. What is a DLCO test, and what are our requirements for an acceptable test and report?
1. A DLCO test measures the gas exchange across cell membranes in your lungs. It measures how well CO diffuses from the alveoli (air sacs) of your lungs into your blood. DLCO may be severely reduced in some disorders, such as interstitial lung disease (for example, idiopathic pulmonary fibrosis, asbestosis, and sarcoidosis) and COPD (particularly emphysema), even when the results of spirometry are not significantly reduced. We use the average of two of your unadjusted (that is, uncorrected for hemoglobin concentration) DLCO measurements reported in mL CO (STPD)/min/mmHg to evaluate your respiratory disorder under 3.02C1.
2. We have the following requirements for DLCO tests under these listings:
a. You must be medically stable at the time of the test. See 3.00E2a.
b. The test must use the single-breath technique.
(i) The VI during the DLCO maneuver must be at least 85 percent of your current FVC, and your time of inhalation must be less than 4 seconds. (See 3.00E for our rules for programmatically acceptable spirometry.) If you do not have an FVC measurement on the same day as the DLCO test, we may use your FVC from programmatically acceptable spirometry administered within 90 days of the DLCO test.
(ii) Your breath-hold time must be between 8 and 12 seconds.
(iii) Your total exhalation time must be less than or equal to 4 seconds, with a sample collection time of less than 3 seconds. If your FVC is at least 2.0 L, the washout volume must be between 0.75 L and 1.0 L. If your FVC is less than 2.0 L, the washout volume must be at least 0.5 L.
3. The DLCO test report must include the following information:
a. The date of the test and your name, age or date of birth, gender, and height without shoes. (We will assume that your recorded height on the date of the test is without shoes, unless we have evidence to the contrary.) If your spine is abnormally curved (for example, you have kyphoscoliosis), we will substitute the longest distance between your outstretched fingertips with your arms abducted 90 degrees in place of your height when this measurement is greater than your standing height without shoes.
b. Any factors, if applicable, that can affect the interpretation of the test results (for example, your cooperation or effort in doing the test).
c. Legible tracings of your VI, breath-hold maneuver, and volume of exhaled gas showing your name and the date of the test for each DLCO maneuver.
d. At least two acceptable (see 3.00F2) DLCO measurements within 3 mL CO (STPD)/min/mmHg of each other or within 10 percent of the highest value.
4. We may need to purchase a DLCO test to determine whether your disorder meets 3.02C1 when we have evidence showing that you have a chronic respiratory disorder that could result in impaired gas exchange, unless we can make a fully favorable determination or decision on another basis. Since the DLCO calculation requires a current FVC measurement, we may also purchase spirometry at the same time as the DLCO test, even if we already have programmatically acceptable spirometry.
5. Before we purchase a DLCO test, a medical consultant (see §§ 404.1616 and 416.1016 of this chapter), preferably one with experience in the care of people with respiratory disorders, must review your case record to determine if we need the test. The medical source we designate to administer the test is solely responsible for deciding whether it is safe for you to do the test and for how to administer it.
G. What is an ABG test, and what are our requirements for an acceptable test and report?
1. General. An ABG test measures PaO2, PaCO2, and the concentration of hydrogen ions in your arterial blood. We use a resting or an exercise ABG measurement to evaluate your respiratory disorder under 3.02C2.
2. Resting ABG tests.
a. We have the following requirements for resting ABG tests under these listings:
(i) You must be medically stable at the time of the test. See 3.00E2a.
(ii) The test must be administered while you are breathing room air; that is, without oxygen supplementation.
b. The resting ABG test report must include the following information:
(i) Your name, the date of the test, and either the altitude or both the city and State of the test site.
(ii) The PaO2 and PaCO2 values.
c. We may need to purchase a resting ABG test to determine whether your disorder meets 3.02C2 when we have evidence showing that you have a chronic respiratory disorder that could result in impaired gas exchange, unless we can make a fully favorable determination or decision on another basis.
d. Before we purchase a resting ABG test, a medical consultant (see §§ 404.1616 and 416.1016 of this chapter), preferably one with experience in the care of people with respiratory disorders, must review your case record to determine if we need the test. The medical source we designate to administer the test is solely responsible for deciding whether it is safe for you to do the test and for how to administer it.
3. Exercise ABG tests.
a. We will not purchase an exercise ABG test.
b. We have the following requirements for exercise ABG tests under these listings:
(i) You must have done the exercise under steady state conditions while breathing room air. If you were tested on a treadmill, you generally must have exercised for at least 4 minutes at a grade and speed providing oxygen (O2) consumption of approximately 17.5 milliliters per kilogram per minute (mL/kg/min) or 5.0 metabolic equivalents (METs). If you were tested on a cycle ergometer, you generally must have exercised for at least 4 minutes at an exercise equivalent of 5.0 METs.
(ii) We may use a test in which you have not exercised for at least 4 minutes. If you were unable to complete at least 4 minutes of steady state exercise, we need a statement by the person administering the test about whether the results are a valid indication of your respiratory status. For example, this statement may include information about your cooperation or effort in doing the test and whether you were limited in completing the test because of your respiratory disorder or another impairment.
c. The exercise ABG test report must include the following information:
(i) Your name, the date of the test, and either the altitude or both the city and state of the test site.
(ii) The PaO2 and PaCO2 values.
H. What is pulse oximetry, and what are our requirements for an acceptable test and report?
1. Pulse oximetry measures SpO2, the percentage of oxygen saturation of blood hemoglobin. We use a pulse oximetry measurement (either at rest, during a 6MWT, or after a 6MWT) to evaluate your respiratory disorder under 3.02C3 or, if you have CF, to evaluate it under 3.04F.
2. We have the following requirements for pulse oximetry under 3.02C3:
a. You must be medically stable at the time of the test. See 3.00E2a.
b. Your pulse oximetry measurement must be recorded while you are breathing room air; that is, without oxygen supplementation.
c. Your pulse oximetry measurement must be stable. By “stable,” we mean that the range of SpO2 values (that is, lowest to highest) during any 15-second interval cannot exceed 2 percentage points. For example: (1) The measurement is stable if the lowest SpO2 value during a 15-second interval is 87 percent and the highest value is 89 percent - a range of 2 percentage points. (2) The measurement is not stable if the lowest value is 86 percent and the highest value is 89 percent - a range of 3 percentage points.
d. If you have had more than one measurement (for example, at rest and after a 6MWT), we will use the measurement with the lowest SpO2 value.
e. The pulse oximetry report must include the following information:
(i) Your name, the date of the test, and either the altitude or both the city and State of the test site.
(ii) A graphical printout showing your SpO2 value and a concurrent, acceptable pulse wave. An acceptable pulse wave is one that shows the characteristic pulse wave; that is, sawtooth-shaped with a rapid systolic upstroke (nearly vertical) followed by a slower diastolic downstroke (angled downward).
f. We may need to purchase pulse oximetry at rest to determine whether your disorder meets 3.02C3 when we have evidence showing that you have a chronic respiratory disorder that could result in impaired gas exchange, unless we can make a fully favorable determination or decision on another basis. We may purchase pulse oximetry during and after a 6MWT if your SpO2 value at rest is greater than the value in Table V.
g. Before we purchase pulse oximetry, a medical consultant (see §§ 404.1616 and 416.1016 of this chapter), preferably one with experience in the care of people with respiratory disorders, must review your case record to determine if we need the test. The medical source we designate to administer the test is solely responsible for deciding whether it is safe for you to do the test and for how to administer it.
3. We have the following requirements for pulse oximetry under 3.04F:
a. You must be medically stable at the time of the test. See 3.00E2a.
b. Your pulse oximetry measurement must be recorded while you are breathing room air; that is, without oxygen supplementation.
c. If you have had more than one measurement (for example, at rest and after a 6MWT), we will use the measurement with the lowest SpO2 value.
d. The pulse oximetry report must include your name, the date of the test, and either the altitude or both the city and State of the test site. If you have CF, we do not require a graphical printout showing your SpO2 value and a concurrent, acceptable pulse wave.
I. What is asthma and how do we evaluate it?
1. Asthma is a chronic inflammatory disorder of the lung airways that we evaluate under 3.02 or 3.03. If you have respiratory failure resulting from chronic asthma (see 3.00N), we will evaluate it under 3.14.
2. For the purposes of 3.03:
a. We need evidence showing that you have listing-level (see Table VI in 3.03A) airflow obstruction at baseline while you are medically stable.
b. The phrase “consider under a disability for 1 year” in 3.03B does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your asthma continues to meet a listing or is otherwise disabling.
c. We determine the onset of your disability based on the facts of your case, but it will be no later than the admission date of your first of three hospitalizations that satisfy the criteria of 3.03B.
J. What is CF and how do we evaluate it?
1. General. We evaluate CF, a genetic disorder that results in abnormal salt and water transport across cell membranes in the lungs, pancreas, and other body organs, under 3.04. We need the evidence described in 3.00J2 to establish that you have CF.
2. Documentation of CF. We need a report signed by a physician (see §§ 404.1513(a) and 416.913(a) of this chapter) showing both a and b:
a. One of the following:
(i) A positive newborn screen for CF; or
(ii) A history of CF in a sibling; or
(iii) Documentation of at least one specific CF phenotype or clinical criterion (for example, chronic sino-pulmonary disease with persistent colonization or infections with typical CF pathogens, pancreatic insufficiency, or salt-loss syndromes); and
b. One of the following definitive laboratory tests:
(i) An elevated sweat chloride concentration equal to or greater than 60 millimoles per L; or
(ii) The identification of two CF gene mutations affecting the CFTR; or
(iii) Characteristic abnormalities in ion transport across the nasal epithelium.
c. When we have the report showing a and b, but it is not signed by a physician, we also need a report from a physician stating that you have CF.
d. When we do not have the report showing a and b, we need a report from a physician that is persuasive that a positive diagnosis of CF was confirmed by an appropriate definitive laboratory test. To be persuasive, this report must include a statement by the physician that you had the appropriate definitive laboratory test for diagnosing CF. The report must provide the test results or explain how your diagnosis was established that is consistent with the prevailing state of medical knowledge and clinical practice.
3. CF pulmonary exacerbations. Examples of CF pulmonary exacerbations include increased cough and sputum production, hemoptysis, increased shortness of breath, increased fatigue, and reduction in pulmonary function. Treatment usually includes intravenous antibiotics and intensified airway clearance therapy (for example, increased frequencies of chest percussion or increased use of inhaled nebulized therapies, such as bronchodilators or mucolytics).
4. For 3.04G, we require any two exacerbations or complications from the list in 3.04G1 through 3.04G4 within a 12-month period. You may have two of the same exacerbation or complication or two different ones.
a. If you have two of the acute exacerbations or complications we describe in 3.04G1 and 3.04G2, there must be at least 30 days between the two.
b. If you have one of the acute exacerbations or complications we describe in 3.04G1 and 3.04G2 and one of the chronic complications we describe in 3.04G3 and 3.04G4, the two can occur during the same time. For example, your CF meets 3.04G if you have the pulmonary hemorrhage we describe in 3.04G2 and the weight loss we describe in 3.04G3 even if the pulmonary hemorrhage occurs during the 90-day period in 3.04G3.
c. Your CF also meets 3.04G if you have both of the chronic complications in 3.04G3 and 3.04G4.
5. CF may also affect other body systems such as digestive or endocrine. If your CF, including pulmonary exacerbations and nonpulmonary complications, does not meet or medically equal a respiratory disorders listing, we may evaluate your CF-related impairments under the listings in the affected body system.
K. What is bronchiectasis and how do we evaluate it? Bronchiectasis is a chronic respiratory disorder that is characterized by abnormal and irreversible dilatation (enlargement) of the airways below the trachea, which may be associated with the accumulation of mucus, bacterial infections, and eventual airway scarring. We require imaging (see 3.00D3) to document this disorder. We evaluate your bronchiectasis under 3.02, or under 3.07 if you are having exacerbations or complications (for example, acute bacterial infections, increased shortness of breath, or coughing up blood) that require hospitalization.
L. What is chronic pulmonary hypertension and how do we evaluate it?
1. Chronic pulmonary hypertension is an increase in the blood pressure of the blood vessels of the lungs. If pulmonary hypertension is not adequately treated, it can eventually result in right heart failure. We evaluate chronic pulmonary hypertension due to any cause under 3.09.
2. Chronic pulmonary hypertension is usually diagnosed by catheterization of the pulmonary artery. We will not purchase cardiac catheterization.
M. How do we evaluate lung transplantation? If you receive a lung transplant (or a lung transplant simultaneously with other organs, such as the heart), we will consider you to be disabled under 3.11 for 3 years from the date of the transplant. After that, we evaluate your residual impairment(s) by considering the adequacy of your post-transplant function, the frequency and severity of any rejection episodes you have, complications in other body systems, and adverse treatment effects. People who receive organ transplants generally have impairments that meet our definition of disability before they undergo transplantation. The phrase “consider under a disability for 3 years” in 3.11 does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your impairment(s) continues to meet a listing or is otherwise disabling. We determine the onset of your disability based on the facts of your case.
N. What is respiratory failure and how do we evaluate it? Respiratory failure is the inability of the lungs to perform their basic function of gas exchange. We evaluate respiratory failure under 3.04D if you have CF-related respiratory failure, or under 3.14 if you have respiratory failure due to any other chronic respiratory disorder. Continuous positive airway pressure does not satisfy the criterion in 3.04D or 3.14, and cannot be substituted as an equivalent finding, for invasive mechanical ventilation or noninvasive ventilation with BiPAP.
O. How do we consider the effects of obesity when we evaluate your respiratory disorder? Obesity is a medically determinable impairment that is often associated with respiratory disorders. Obesity makes it harder for the chest and lungs to expand, which can compromise the ability of the respiratory system to supply adequate oxygen to the body. The combined effects of obesity with a respiratory disorder can be greater than the effects of each of the impairments considered separately. We consider any additional and cumulative effects of your obesity when we determine whether you have a severe respiratory disorder, a listing-level respiratory disorder, a combination of impairments that medically equals the severity of a listed impairment, and when we assess your residual functional capacity.
P. What are sleep-related breathing disorders and how do we evaluate them?
1. Sleep-related breathing disorders (for example, sleep apnea) are characterized by transient episodes of interrupted breathing during sleep, which disrupt normal sleep patterns. Prolonged episodes can result in disorders such as hypoxemia (low blood oxygen) and pulmonary vasoconstriction (restricted blood flow in pulmonary blood vessels). Over time, these disorders may lead to chronic pulmonary hypertension or other complications.
2. We evaluate the complications of sleep-related breathing disorders under the listings in the affected body system(s). For example, we evaluate chronic pulmonary hypertension due to any cause under 3.09; chronic heart failure under 4.02; and disturbances in mood, cognition, and behavior under 12.02 or another appropriate mental disorders listing. We will not purchase polysomnography (sleep study).
Q. How do we evaluate mycobacterial, mycotic, and other chronic infections of the lungs? We evaluate chronic infections of the lungs that result in limitations in your respiratory function under 3.02.
R. How do we evaluate respiratory disorders that do not meet one of these listings?
1. These listings are only examples of common respiratory disorders that we consider severe enough to prevent you from doing any gainful activity. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that meets the criteria of a listing in another body system. For example, if your CF has resulted in chronic pancreatic or hepatobiliary disease, we evaluate your impairment under the listings in 5.00.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. See §§ 404.1526 and 416.926 of this chapter. Respiratory disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not meet or medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. We proceed to the fourth step and, if necessary, the fifth step of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
3.01 Category of Impairments, Respiratory Disorders3.02 Chronic respiratory disorders due to any cause except CF (for CF, see 3.04) with A, B, C, or D:
A. FEV1 (see 3.00E) less than or equal to the value in Table I-A or I-B for your age, gender, and height without shoes (see 3.00E3a).
Table I - FEV1 Criteria for 3.02A
| Height without
shoes (centimeters) < means less than |
Height without
shoes (inches) < means less than |
Table I-A | Table I-B | ||
|---|---|---|---|---|---|
| Age 18 to attainment of age 20 | Age 20 or older | ||||
| Females FEV1 less than or
equal to (L, BTPS) |
Males FEV1 less than or equal
to (L, BTPS) |
Females FEV1 less than or
equal to (L, BTPS) |
Males FEV1 less than or equal
to (L, BTPS) |
||
| <153.0 | <60.25 | 1.20 | 1.45 | 1.05 | 1.20 |
| 153.0 to <159.0 | 60.25 to <62.50 | 1.30 | 1.55 | 1.15 | 1.35 |
| 159.0 to <164.0 | 62.50 to <64.50 | 1.40 | 1.65 | 1.25 | 1.40 |
| 164.0 to <169.0 | 64.50 to <66.50 | 1.45 | 1.75 | 1.35 | 1.50 |
| 169.0 to <174.0 | 66.50 to <68.50 | 1.55 | 1.85 | 1.45 | 1.60 |
| 174.0 to <180.0 | 68.50 to <70.75 | 1.65 | 2.00 | 1.55 | 1.75 |
| 180.0 to <185.0 | 70.75 to <72.75 | 1.75 | 2.10 | 1.65 | 1.85 |
| 185.0 or more | 72.75 or more | 1.80 | 2.15 | 1.70 | 1.90 |
OR
B. FVC (see 3.00E) less than or equal to the value in Table II-A or II-B for your age, gender, and height without shoes (see 3.00E3a).
| Height without
shoes (centimeters) < means less than |
Height without
shoes (inches) < means less than |
Table II-A | Table II-B | ||
|---|---|---|---|---|---|
| Age 18 to attainment of age 20 | Age 20 or older | ||||
| Females FVC less than or
equal to (L, BTPS) |
Males FVC less than or equal
to (L, BTPS) |
Females FVC less than or
equal to (L, BTPS) |
Males FVC less than or equal
to (L, BTPS) |
||
| <153.0 | <60.25 | 1.35 | 1.65 | 1.30 | 1.50 |
| 153.0 to <159.0 | 60.25 to <62.50 | 1.50 | 1.80 | 1.40 | 1.65 |
| 159.0 to <164.0 | 62.50 to <64.50 | 1.60 | 1.90 | 1.50 | 1.75 |
| 164.0 to <169.0 | 64.50 to <66.50 | 1.70 | 2.05 | 1.60 | 1.90 |
| 169.0 to <174.0 | 66.50 to <68.50 | 1.80 | 2.20 | 1.70 | 2.00 |
| 174.0 to <180.0 | 68.50 to <70.75 | 1.90 | 2.35 | 1.85 | 2.20 |
| 180.0 to <185.0 | 70.75 to <72.75 | 2.05 | 2.50 | 1.95 | 2.30 |
| 185.0 or more | 72.75 or more | 2.10 | 2.60 | 2.00 | 2.40 |
OR
C. Chronic impairment of gas exchange demonstrated by 1, 2, or 3:
1. Average of two unadjusted, single-breath DLCO measurements (see 3.00F) less than or equal to the value in Table III for your gender and height without shoes (see 3.00F3a); or
Table III - DLCO Criteria for 3.02C1
| Height without shoes (centimeters) < means less than |
Height without shoes (inches) < means less than |
Females DLCO less than or
equal to (mL CO (STPD)/min/mmHg) |
Males DLCO less than or equal
to (mL CO (STPD)/min/mmHg) |
|---|---|---|---|
| <153.0 | < 60.25 | 8.0 | 9.0 |
| 153.0 to <159.0 | 60.25 to <62.50 | 8.5 | 9.5 |
| 159.0 to <164.0 | 62.50 to <64.50 | 9.0 | 10.0 |
| 164.0 to <169.0 | 64.50 to <66.50 | 9.5 | 10.5 |
| 169.0 to <174.0 | 66.50 to <68.50 | 10.0 | 11.0 |
| 174.0 to <180.0 | 68.50 to <70.75 | 10.5 | 11.5 |
| 180.0 to <185.0 | 70.75 to <72.75 | 11.0 | 12.0 |
| 185.0 or more | 72.75 or more | 11.5 | 12.5 |
2. Arterial PaO2 and PaCO2 measured concurrently by an ABG test, while at rest or during steady state exercise, breathing room air (see 3.00G3b), less than or equal to the applicable values in Table IV-A, IV-B, or IV-C; or
Tables IV-A, IV-B, and IV-C - ABG Criteria for 3.02C2Table IV-A
[Applicable at test sites less than 3,000 feet above sea level]
| Arterial PaCO2 (mm Hg) and | Arterial PaO2 less than or
equal to (mm Hg) |
|---|---|
| 30 or below | 65 |
| 31 | 64 |
| 32 | 63 |
| 33 | 62 |
| 34 | 61 |
| 35 | 60 |
| 36 | 59 |
| 37 | 58 |
| 38 | 57 |
| 39 | 56 |
| 40 or above | 55 |
Table IV-B
[Applicable at test sites from 3,000 through 6,000 feet above sea level]
| Arterial PaCO2 (mm Hg) and | Arterial PaO2 less than or
equal to (mm Hg) |
|---|---|
| 30 or below | 60 |
| 31 | 59 |
| 32 | 58 |
| 33 | 57 |
| 34 | 56 |
| 35 | 55 |
| 36 | 54 |
| 37 | 53 |
| 38 | 52 |
| 39 | 51 |
| 40 or above | 50 |
Table IV-C
[Applicable at test sites over 6,000 feet above sea level]
| Arterial PaCO2 (mm Hg) and | Arterial PaO2 less than or
equal to (mm Hg) |
|---|---|
| 30 or below | 55 |
| 31 | 54 |
| 32 | 53 |
| 33 | 52 |
| 34 | 51 |
| 35 | 50 |
| 36 | 49 |
| 37 | 48 |
| 38 | 47 |
| 39 | 46 |
| 40 or above | 45 |
3. SpO2 measured by pulse oximetry (see 3.00H2) either at rest, during a 6MWT, or after a 6MWT, less than or equal to the value in Table V.
Table V - SpO2 Criteria for 3.02C3
| Test site altitude (feet above sea level) |
SpO2 less than or equal to |
|---|---|
| Less than 3,000 | 87 percent. |
| 3,000 through 6,000 | 85 percent. |
| Over 6,000 | 83 percent. |
OR
D. Exacerbations or complications requiring three hospitalizations within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
3.03 Asthma (see 3.00I), with both A and B:
A. FEV1 (see 3.00E1) less than or equal to the value in Table VI-A or VI-B for your age, gender, and height without shoes (see 3.00E3a) measured within the same 12-month period as the hospitalizations in 3.03B.
Table VI - FEV1 Criteria for 3.03A
| Height without
shoes (centimeters) < means less than |
Height without
shoes (inches) < means less than |
Table VI-A | Table VI-B | ||
|---|---|---|---|---|---|
| Age 18 to attainment of age 20 | Age 20 or older | ||||
| Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
||
| <153.0 | <60.25 | 1.65 | 1.90 | 1.45 | 1.60 |
| 153.0 to <159.0 | 60.25 to <62.50 | 1.75 | 2.05 | 1.55 | 1.75 |
| 159.0 to <164.0 | 62.50 to <64.50 | 1.85 | 2.15 | 1.65 | 1.90 |
| 164.0 to <169.0 | 64.50 to <66.50 | 1.95 | 2.30 | 1.75 | 2.00 |
| 169.0 to <174.0 | 66.50 to <68.50 | 2.05 | 2.45 | 1.85 | 2.15 |
| 174.0 to <180.0 | 68.50 to <70.75 | 2.20 | 2.60 | 2.00 | 2.30 |
| 180.0 to <185.0 | 70.75 to <72.75 | 2.35 | 2.75 | 2.10 | 2.45 |
| 185.0 or more | 72.75 or more | 2.40 | 2.85 | 2.20 | 2.55 |
AND
B. Exacerbations or complications requiring three hospitalizations within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization. Consider under a disability for 1 year from the discharge date of the last hospitalization; after that, evaluate the residual impairment(s) under 3.03 or another appropriate listing.
3.04 Cystic fibrosis (documented as described in 3.00J2) with A, B, C, D, E, F, or G:
A. FEV1 (see 3.00E) less than or equal to the value in Table VII-A or VII-B for your age, gender, and height without shoes (see 3.00E3a).
Table VII - FEV1 Criteria for 3.04A
| Height without
shoes (centimeters) < means less than |
Height without
shoes (inches) < means less than |
Table VII-A | Table VII-B | ||
|---|---|---|---|---|---|
| Age 18 to attainment of age 20 | Age 20 or older | ||||
| Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
||
| <153.0 | <60.25 | 1.65 | 1.90 | 1.45 | 1.60 |
| 153.0 to <159.0 | 60.25 to <62.50 | 1.75 | 2.05 | 1.55 | 1.75 |
| 159.0 to <164.0 | 62.50 to <64.50 | 1.85 | 2.15 | 1.65 | 1.90 |
| 164.0 to <169.0 | 64.50 to <66.50 | 1.95 | 2.30 | 1.75 | 2.00 |
| 169.0 to <174.0 | 66.50 to <68.50 | 2.05 | 2.45 | 1.85 | 2.15 |
| 174.0 to <180.0 | 68.50 to <70.75 | 2.20 | 2.60 | 2.00 | 2.30 |
| 180.0 to <185.0 | 70.75 to <72.75 | 2.35 | 2.75 | 2.10 | 2.45 |
| 185.0 or more | 72.75 or more | 2.40 | 2.85 | 2.20 | 2.55 |
OR
B. Exacerbations or complications (see 3.00J3) requiring three hospitalizations of any length within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review).
OR
C. Spontaneous pneumothorax, secondary to CF, requiring chest tube placement.
OR
D. Respiratory failure (see 3.00N) requiring invasive mechanical ventilation, noninvasive ventilation with BiPAP, or a combination of both treatments, for a continuous period of at least 48 hours, or for a continuous period of at least 72 hours if postoperatively.
OR
E. Pulmonary hemorrhage requiring vascular embolization to control bleeding.
OR
F. SpO2 measured by pulse oximetry (see 3.00H3) either at rest, during a 6MWT, or after a 6MWT, less than or equal to the value in Table VIII, twice within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review).
Tables VIII - SpO2 Criteria for 3.04F
| Test site altitude (feet above sea level) |
SpO2 less than or equal to |
|---|---|
| Less than 3,000 | 89 percent. |
| 3,000 through 6,000 | 87 percent. |
| Over 6,000 | 85 percent. |
OR
G. Two of the following exacerbations or complications (either two of the same or two different, see 3.00J3 and 3.00J4) within a 12-month period (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review):
1. Pulmonary exacerbation requiring 10 consecutive days of intravenous antibiotic treatment.
2. Pulmonary hemorrhage (hemoptysis with more than blood-streaked sputum but not requiring vascular embolization) requiring hospitalization of any length.
3. Weight loss requiring daily supplemental enteral nutrition via a gastrostomy for at least 90 consecutive days or parenteral nutrition via a central venous catheter for at least 90 consecutive days.
4. CFRD requiring daily insulin therapy for at least 90 consecutive days.
3.05 [Reserved]
3.06 [Reserved]
3.07 Bronchiectasis (see 3.00K), documented by imaging (see 3.00D3), with exacerbations or complications requiring three hospitalizations within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
3.08 [Reserved]
3.09 Chronic pulmonary hypertension due to any cause (see 3.00L) documented by mean pulmonary artery pressure equal to or greater than 40 mm Hg as determined by cardiac catheterization while medically stable (see 3.00E2a).
3.10 [Reserved]
3.11 Lung transplantation (see 3.00M). Consider under a disability for 3 years from the date of the transplant; after that, evaluate the residual impairment(s).
3.12 [Reserved]
3.13 [Reserved]
3.14 Respiratory failure (see 3.00N) resulting from any underlying chronic respiratory disorder except CF (for CF, see 3.04D), requiring invasive mechanical ventilation, noninvasive ventilation with BiPAP, or a combination of both treatments, for a continuous period of at least 48 hours, or for a continuous period of at least 72 hours if postoperatively, twice within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review).
4.00 Cardiovascular System A. General1. What do we mean by a cardiovascular impairment?
a. We mean any disorder that affects the proper functioning of the heart or the circulatory system (that is, arteries, veins, capillaries, and the lymphatic drainage). The disorder can be congenital or acquired.
b. Cardiovascular impairment results from one or more of four consequences of heart disease:
(i) Chronic heart failure or ventricular dysfunction.
(ii) Discomfort or pain due to myocardial ischemia, with or without necrosis of heart muscle.
(iii) Syncope, or near syncope, due to inadequate cerebral perfusion from any cardiac cause, such as obstruction of flow or disturbance in rhythm or conduction resulting in inadequate cardiac output.
(iv) Central cyanosis due to right-to-left shunt, reduced oxygen concentration in the arterial blood, or pulmonary vascular disease.
c. Disorders of the veins or arteries (for example, obstruction, rupture, or aneurysm) may cause impairments of the lower extremities (peripheral vascular disease), the central nervous system, the eyes, the kidneys, and other organs. We will evaluate peripheral vascular disease under 4.11 or 4.12 and impairments of another body system(s) under the listings for that body system(s).
2. What do we consider in evaluating cardiovascular impairments? The listings in this section describe cardiovascular impairments based on symptoms, signs, laboratory findings, response to a regimen of prescribed treatment, and functional limitations.
3. What do the following terms or phrases mean in these listings?
a. Medical consultant is an individual defined in §§ 404.1616(a) and 416.1016(a). This term does not include medical sources who provide consultative examinations for us. We use the abbreviation “MC” throughout this section to designate a medical consultant.
b. Persistent means that the longitudinal clinical record shows that, with few exceptions, the required finding(s) has been present, or is expected to be present, for a continuous period of at least 12 months, such that a pattern of continuing severity is established.
c. Recurrent means that the longitudinal clinical record shows that, within a consecutive 12-month period, the finding(s) occurs at least three times, with intervening periods of improvement of sufficient duration that it is clear that separate events are involved.
d. Appropriate medically acceptable imaging means that the technique used is the proper one to evaluate and diagnose the impairment and is commonly recognized as accurate for assessing the cited finding.
e. A consecutive 12-month period means a period of 12 consecutive months, all or part of which must occur within the period we are considering in connection with an application or continuing disability review.
f. Uncontrolled means the impairment does not adequately respond to standard prescribed medical treatment.
B. Documenting Cardiovascular Impairment1. What basic documentation do we need? We need sufficiently detailed reports of history, physical examinations, laboratory studies, and any prescribed treatment and response to allow us to assess the severity and duration of your cardiovascular impairment. A longitudinal clinical record covering a period of not less than 3 months of observations and treatment is usually necessary, unless we can make a determination or decision based on the current evidence.
2. Why is a longitudinal clinical record important? We will usually need a longitudinal clinical record to assess the severity and expected duration of your impairment(s). If you have a listing-level impairment, you probably will have received medically prescribed treatment. Whenever there is evidence of such treatment, your longitudinal clinical record should include a description of the ongoing management and evaluation provided by your treating or other medical source. It should also include your response to this medical management, as well as information about the nature and severity of your impairment. The record will provide us with information on your functional status over an extended period of time and show whether your ability to function is improving, worsening, or unchanging.
3. What if you have not received ongoing medical treatment?
a. You may not have received ongoing treatment or have an ongoing relationship with the medical community despite the existence of a severe impairment(s). In this situation, we will base our evaluation on the current objective medical evidence and the other evidence we have. If you do not receive treatment, you cannot show an impairment that meets the criteria of most of these listings. However, we may find you disabled because you have another impairment(s) that in combination with your cardiovascular impairment medically equals the severity of a listed impairment or based on consideration of your residual functional capacity and age, education, and work experience.
b. Unless we can decide your claim favorably on the basis of the current evidence, a longitudinal record is still important. In rare instances where there is no or insufficient longitudinal evidence, we may purchase a consultative examination(s) to help us establish the severity and duration of your impairment.
4. When will we wait before we ask for more evidence?
a. We will wait when we have information showing that your impairment is not yet stable and the expected change in your impairment might affect our determination or decision. In these situations, we need to wait to properly evaluate the severity and duration of your impairment during a stable period. Examples of when we might wait are:
(i) If you have had a recent acute event; for example, a myocardial infarction (heart attack).
(ii) If you have recently had a corrective cardiac procedure; for example, coronary artery bypass grafting.
(iii) If you have started new drug therapy and your response to this treatment has not yet been established; for example, beta-blocker therapy for dilated congestive cardiomyopathy.
b. In these situations, we will obtain more evidence 3 months following the event before we evaluate your impairment. However, we will not wait if we have enough information to make a determination or decision based on all of the relevant evidence in your case.
5. Will we purchase any studies? In appropriate situations, we will purchase studies necessary to substantiate the diagnosis or to document the severity of your impairment, generally after we have evaluated the medical and other evidence we already have. We will not purchase studies involving exercise testing if there is significant risk involved or if there is another medical reason not to perform the test. We will follow sections 4.00C6, 4.00C7, and 4.00C8 when we decide whether to purchase exercise testing.
6. What studies will we not purchase? We will not purchase any studies involving cardiac catheterization, such as coronary angiography, arteriograms, or electrophysiological studies. However, if the results of catheterization are part of the existing evidence we have, we will consider them together with the other relevant evidence. See 4.00C15a.
C. Using Cardiovascular Test Results1. What is an ECG?
a. ECG stands for electrocardiograph or electrocardiogram. An electrocardiograph is a machine that records electrical impulses of your heart on a strip of paper called an electrocardiogram or a tracing. To record the ECG, a technician positions a number of small contacts (or leads) on your arms, legs, and across your chest to connect them to the ECG machine. An ECG may be done while you are resting or exercising.
b. The ECG tracing may indicate that you have a heart abnormality. It may indicate that your heart muscle is not getting as much oxygen as it needs (ischemia), that your heart rhythm is abnormal (arrhythmia), or that there are other abnormalities of your heart, such as left ventricular enlargement.
2. How do we evaluate ECG evidence? We consider a number of factors when we evaluate ECG evidence:
a. An original or legible copy of the 12-lead ECG obtained at rest must be appropriately dated and labeled, with the standardization inscribed on the tracing. Alteration in standardization of specific leads (such as to accommodate large QRS amplitudes) must be identified on those leads.
(i) Detailed descriptions or computer-averaged signals without original or legible copies of the ECG as described in listing 4.00C2a are not acceptable.
(ii) The effects of drugs or electrolyte abnormalities must be considered as possible noncardiac causes of ECG abnormalities of ventricular repolarization; that is, those involving the ST segment and T wave. If available, the predrug (especially digitalis glycosides) ECG should be submitted.
b. ECGs obtained in conjunction with treadmill, bicycle, or arm exercise tests should meet the following specifications:
(i) ECG reports must include the original calibrated ECG tracings or a legible copy.
(ii) A 12-lead baseline ECG must be recorded in the upright position before exercise.
(iii) A 12-lead ECG should be recorded at the end of each minute of exercise.
(iv) If ECG documentation of the effects of hyperventilation is obtained, the exercise test should be deferred for at least 10 minutes because metabolic changes of hyperventilation may alter the physiologic and ECG-recorded response to exercise.
(v) Post-exercise ECGs should be recorded using a generally accepted protocol consistent with the prevailing state of medical knowledge and clinical practice.
(vi) All resting, exercise, and recovery ECG strips must have the standardization inscribed on the tracing. The ECG strips should be labeled to indicate the date, the times recorded and the relationship to the stage of the exercise protocol. The speed and grade (treadmill test) or work rate (bicycle or arm ergometric test) should be recorded. The highest level of exercise achieved, heart rate and blood pressure levels during testing, and the reason(s) for terminating the test (including limiting signs or symptoms) must be recorded.
3. What are exercise tests and what are they used for?
a. Exercise tests have you perform physical activity and record how your cardiovascular system responds. Exercise tests usually involve walking on a treadmill, but other forms of exercise, such as an exercise bicycle or an arm exercise machine, may be used. Exercise testing may be done for various reasons; such as to evaluate the severity of your coronary artery disease or peripheral vascular disease or to evaluate your progress after a cardiac procedure or an acute event, like a myocardial infarction (heart attack). Exercise testing is the most widely used testing for identifying the presence of myocardial ischemia and for estimating maximal aerobic capacity (usually expressed in METs - metabolic equivalents) if you have heart disease.
b. We include exercise tolerance test (ETT) criteria in 4.02B3 (chronic heart failure) and 4.04A (ischemic heart disease). To meet the ETT criteria in these listings, the ETT must be a sign-or symptom-limited test in which you exercise while connected to an ECG until you develop a sign or symptom that indicates that you have exercised as much as is considered safe for you.
c. In 4.12B, we also refer to exercise testing for peripheral vascular disease. In this test, you walk on a treadmill, usually for a specified period of time, and the individual who administers the test measures the effect of exercise on the flow of blood in your legs, usually by using ultrasound. The test is also called an exercise Doppler test. Even though this test is intended to evaluate peripheral vascular disease, it will be stopped for your safety if you develop abnormal signs or symptoms because of heart disease.
d. Each type of test is done in a certain way following specific criteria, called a protocol. For our program, we also specify certain aspects of how any exercise test we purchase is to be done. See 4.00C10 and 4.00C17.
4. Do ETTs have limitations? An ETT provides an estimate of aerobic capacity for walking on a grade, bicycling, or moving one's arms in an environmentally controlled setting. Therefore, ETT results do not correlate with the ability to perform other types of exertional activities, such as lifting and carrying heavy loads, and do not provide an estimate of the ability to perform activities required for work in all possible work environments or throughout a workday. Also, certain medications (such as beta blockers) and conduction disorders (such as left or right bundle branch blocks) can cause false-negative or false-positive results. Therefore, we must consider the results of an ETT together with all the other relevant evidence in your case record.
5. How does an ETT with measurement of maximal or peak oxygen uptake VO2) differ from other ETTs? Occasionally, medical evidence will include the results of an ETT with VO2 While ETTs without measurement of VO2 provide only an estimate of aerobic capacity, measured maximal or peak oxygen uptake provides an accurate measurement of aerobic capacity, which is often expressed in METs (metabolic equivalents). The MET level may not be indicated in the report of attained maximal or peak VO2 testing, but can be calculated as follows: 1 MET = 3.5 milliliters (ml) of oxygen uptake per kilogram (kg) of body weight per minute. For example, a 70 kg (154 lb.) individual who achieves a maximal or peak VO2 of 1225 ml in 1 minute has attained 5 METs (1225 ml/70 kg/1 min = 17.5 ml/kg/min. 17.5/3.5 = 5 METs).
6. When will we consider whether to purchase an exercise test?
a. We will consider whether to purchase an exercise test when:
(i) There is a question whether your cardiovascular impairment meets or medically equals the severity of one of the listings, or there is no timely test in the evidence we have (see 4.00C9), and we cannot find you disabled on some other basis; or
(ii) We need to assess your residual functional capacity and there is insufficient evidence in the record to make a determination or decision.
b. We will not purchase an exercise test when we can make our determination or decision based on the evidence we already have.
7. What must we do before purchasing an exercise test?
a. Before we purchase an exercise test, an MC, preferably one with experience in the care of patients with cardiovascular disease, must review the pertinent history, physical examinations, and laboratory tests that we have to determine whether the test would present a significant risk to you or if there is some other medical reason not to purchase the test (see 4.00C8).
b. If you are under the care of a treating source (see §§ 404.1502 and 416.902) for a cardiovascular impairment, this source has not performed an exercise test, and there are no reported significant risks to testing, we will request a statement from that source explaining why it was not done or should not be done before we decide whether we will purchase the test.
c. The MC, in accordance with the regulations and other instructions on consultative examinations, will generally give great weight to the treating source's opinion about the risk of exercise testing to you and will generally not override it. In the rare situation in which the MC does override the treating source's opinion, the MC must prepare a written rationale documenting the reasons for overriding the opinion.
d. If you do not have a treating source or we cannot obtain a statement from your treating source, the MC is responsible for assessing the risk to exercise testing based on a review of the records we have before purchasing an exercise test for you.
e. We must also provide your records to the medical source who performs the exercise test for review prior to conducting the test if the source does not already have them. The medical source who performs the exercise test has the ultimate responsibility for deciding whether you would be at risk.
8. When will we not purchase an exercise test or wait before we purchase an exercise test?
a. We will not purchase an exercise test when an MC finds that you have one of the following significant risk factors:
(i) Unstable angina not previously stabilized by medical treatment.
(ii) Uncontrolled cardiac arrhythmias causing symptoms or hemodynamic compromise.
(iii) An implanted cardiac defibrillator.
(iv) Symptomatic severe aortic stenosis.
(v) Uncontrolled symptomatic heart failure.
(vi) Aortic dissection.
(vii) Severe pulmonary hypertension (pulmonary artery systolic pressure greater than 60 mm Hg).
(viii) Left main coronary stenosis of 50 percent or greater that has not been bypassed.
(ix) Moderate stenotic valvular disease with a systolic gradient across the aortic valve of 50 mm Hg or greater.
(x) Severe arterial hypertension (systolic greater than 200 mm Hg or diastolic greater than 110 mm Hg).
(xi) Hypertrophic cardiomyopathy with a systolic gradient of 50 mm Hg or greater.
b. We also will not purchase an exercise test when you are prevented from performing exercise testing due to another impairment affecting your ability to use your arms and legs.
c. We will not purchase an ETT to document the presence of a cardiac arrhythmia.
d. We will wait to purchase an exercise test until 3 months after you have had one of the following events. This will allow for maximal, attainable restoration of functional capacity.
(i) Acute myocardial infarction.
(ii) Surgical myocardial revascularization (bypass surgery).
(iii) Other open-heart surgical procedures.
(iv) Percutaneous transluminal coronary angioplasty with or without stenting.
e. If you are deconditioned after an extended period of bedrest or inactivity and could improve with activity, or if you are in acute heart failure and are expected to improve with treatment, we will wait an appropriate period of time for you to recuperate before we purchase an exercise test.
9. What do we mean by a “timely” test?
a. We consider exercise test results to be timely for 12 months after the date they are performed, provided there has been no change in your clinical status that may alter the severity of your cardiovascular impairment.
b. However, an exercise test that is older than 12 months, especially an abnormal one, can still provide information important to our adjudication. For example, a test that is more than 12 months old can provide evidence of ischemic heart disease or peripheral vascular disease, information on decreased aerobic capacity, or information about the duration or onset of your impairment. Such tests can be an important component of the longitudinal record.
c. When we evaluate a test that is more than 12 months old, we must consider the results in the context of all the relevant evidence, including why the test was performed and whether there has been an intervening event or improvement or worsening of your impairment.
d. We will purchase a new exercise test only if we cannot make a determination or decision based on the evidence we have.
10. How must ETTs we purchase be performed?
a. The ETT must be a sign- or symptom-limited test characterized by a progressive multistage regimen. It must be performed using a generally accepted protocol consistent with the prevailing state of medical knowledge and clinical practice. A description of the protocol that was followed must be provided, and the test must meet the requirements of 4.00C2b and this section. A radionuclide perfusion scan may be useful for detecting or confirming ischemia when resting ECG abnormalities, medications, or other factors may decrease the accuracy of ECG interpretation of ischemia. (The perfusion imaging is done at the termination of exercise, which may be at a higher MET level than that at which ischemia first occurs. If the imaging confirms the presence of reversible ischemia, the exercise ECG may be useful for detecting the MET level at which ischemia initially appeared.) Exercise tests may also be performed using echocardiography to detect stress-induced ischemia and left ventricular dysfunction (see 4.00C12 and 4.00C13).
b. The exercise test must be paced to your capabilities and be performed following the generally accepted standards for adult exercise test laboratories. With a treadmill test, the speed, grade (incline), and duration of exercise must be recorded for each exercise test stage performed. Other exercise test protocols or techniques should use similar workloads. The exercise protocol may need to be modified in individual cases to allow for a lower initial workload with more slowly graded increments than the standard Bruce protocol.
c. Levels of exercise must be described in terms of workload and duration of each stage; for example, treadmill speed and grade, or bicycle ergometer work rate in kpm/min or watts.
d. The exercise laboratory's physical environment, staffing, and equipment must meet the generally accepted standards for adult exercise test laboratories.
11. How do we evaluate ETT results? We evaluate ETT results on the basis of the work level at which the test becomes abnormal, as documented by onset of signs or symptoms and any ECG or imaging abnormalities. The absence of an ischemic response on an ETT alone does not exclude the diagnosis of ischemic heart disease. We must consider the results of an ETT in the context of all of the other evidence in your case record.
12. When are ETTs done with imaging? When resting ECG abnormalities preclude interpretation of ETT tracings relative to ischemia, a radionuclide (for example, thallium-201 or technetium-99m) perfusion scan or echocardiography in conjunction with an ETT provides better results. You may have resting ECG abnormalities when you have a conduction defect - for example, Wolff-Parkinson-White syndrome, left bundle branch block, left ventricular hypertrophy - or when you are taking digitalis or other antiarrhythmic drugs, or when resting ST changes are present. Also, these techniques can provide a reliable estimate of ejection fraction.
13. Will we purchase ETTs with imaging? We may purchase an ETT with imaging in your case after an MC, preferably one with experience in the care of patients with cardiovascular disease, has reviewed your medical history and physical examination, any report(s) of appropriate medically acceptable imaging, ECGs, and other appropriate tests. We will consider purchasing an ETT with imaging when other information we have is not adequate for us to assess whether you have severe ventricular dysfunction or myocardial ischemia, there is no significant risk involved (see 4.00C8a), and we cannot make our determination or decision based on the evidence we already have.
14. What are drug-induced stress tests? These tests are designed primarily to provide evidence about myocardial ischemia or prior myocardial infarction, but do not require you to exercise. These tests are used when you cannot exercise or cannot exercise enough to achieve the desired cardiac stress. Drug-induced stress tests can also provide evidence about heart chamber dimensions and function; however, these tests do not provide information about your aerobic capacity and cannot be used to help us assess your ability to function. Some of these tests use agents, such as Persantine or adenosine, that dilate the coronary arteries and are used in combination with nuclear agents, such as thallium or technetium (for example, Cardiolyte or Myoview), and a myocardial scan. Other tests use agents, such as dobutamine, that stimulate the heart to contract more forcefully and faster to simulate exercise and are used in combination with a 2-dimensional echocardiogram. We may, when appropriate, purchase a drug-induced stress test to confirm the presence of myocardial ischemia after a review of the evidence in your file by an MC, preferably one with experience in the care of patients with cardiovascular disease.
15. How do we evaluate cardiac catheterization evidence?
a. We will not purchase cardiac catheterization; however, if you have had catheterization, we will make every reasonable effort to obtain the report and any ancillary studies. We will consider the quality and type of data provided and its relevance to the evaluation of your impairment. For adults, we generally see two types of catheterization reports: Coronary arteriography and left ventriculography.
b. For coronary arteriography, the report should provide information citing the method of assessing coronary arterial lumen diameter and the nature and location of obstructive lesions. Drug treatment at baseline and during the procedure should be reported. Some individuals with significant coronary atherosclerotic obstruction have collateral vessels that supply the myocardium distal to the arterial obstruction so that there is no evidence of myocardial damage or ischemia, even with exercise. When the results of quantitative computer measurements and analyses are included in your case record, we will consider them in interpreting the severity of stenotic lesions.
c. For left ventriculography, the report should describe the wall motion of the myocardium with regard to any areas of hypokinesis (abnormally decreased motion), akinesis (lack of motion), or dyskinesis (distortion of motion), and the overall contraction of the ventricle as measured by the ejection fraction. Measurement of chamber volumes and pressures may be useful. Quantitative computer analysis provides precise measurement of segmental left ventricular wall thickness and motion. There is often a poor correlation between left ventricular function at rest and functional capacity for physical activity.
16. What details should exercise Doppler test reports contain? The reports of exercise Doppler tests must describe the level of exercise; for example, the speed and grade of the treadmill settings, the duration of exercise, symptoms during exercise, and the reasons for stopping exercise if the expected level of exercise was not attained. They must also include the blood pressures at the ankle and other pertinent sites measured after exercise and the time required for the systolic blood pressure to return toward or to the pre-exercise level. The graphic tracings, if available, should also be included with the report. All tracings must be annotated with the standardization used by the testing facility.
17. How must exercise Doppler tests we purchase be performed? When we purchase an exercise Doppler test, you must exercise on a treadmill at 2 mph on a 12 percent grade for up to 5 minutes. The reports must include the information specified in 4.00C16. Because this is an exercise test, we must evaluate whether such testing would put you at significant risk, in accordance with the guidance found in 4.00C6, 4.00C7, and 4.00C8.
D. Evaluating Chronic Heart Failure1. What is chronic heart failure (CHF)?
a. CHF is the inability of the heart to pump enough oxygenated blood to body tissues. This syndrome is characterized by symptoms and signs of pulmonary or systemic congestion (fluid retention) or limited cardiac output. Certain laboratory findings of cardiac functional and structural abnormality support the diagnosis of CHF. There are two main types of CHF:
(i) Predominant systolic dysfunction (the inability of the heart to contract normally and expel sufficient blood), which is characterized by a dilated, poorly contracting left ventricle and reduced ejection fraction (abbreviated EF, it represents the percentage of the blood in the ventricle actually pumped out with each contraction), and
(ii) Predominant diastolic dysfunction (the inability of the heart to relax and fill normally), which is characterized by a thickened ventricular muscle, poor ability of the left ventricle to distend, increased ventricular filling pressure, and a normal or increased EF.
b. CHF is considered in these listings as a single category whether due to atherosclerosis (narrowing of the arteries), cardiomyopathy, hypertension, or rheumatic, congenital, or other heart disease. However, if the CHF is the result of primary pulmonary hypertension secondary to disease of the lung (cor pulmonale), we will evaluate your impairment using 3.09, in the respiratory system listings.
2. What evidence of CHF do we need?
a. Cardiomegaly or ventricular dysfunction must be present and demonstrated by appropriate medically acceptable imaging, such as chest x-ray, echocardiography (M-Mode, 2-dimensional, and Doppler), radionuclide studies, or cardiac catheterization.
(i) Abnormal cardiac imaging showing increased left ventricular end diastolic diameter (LVEDD), decreased EF, increased left atrial chamber size, increased ventricular filling pressures measured at cardiac catheterization, or increased left ventricular wall or septum thickness, provides objective measures of both left ventricular function and structural abnormality in heart failure.
(ii) An LVEDD greater than 6.0 cm or an EF of 30 percent or less measured during a period of stability (that is, not during an episode of acute heart failure) may be associated clinically with systolic failure.
(iii) Left ventricular posterior wall thickness added to septal thickness totaling 2.5 cm or greater with left atrium enlarged to 4.5 cm or greater may be associated clinically with diastolic failure.
(iv) However, these measurements alone do not reflect your functional capacity, which we evaluate by considering all of the relevant evidence. In some situations, we may need to purchase an ETT to help us assess your functional capacity.
(v) Other findings on appropriate medically acceptable imaging may include increased pulmonary vascular markings, pleural effusion, and pulmonary edema. These findings need not be present on each report, since CHF may be controlled by prescribed treatment.
b. To establish that you have chronic heart failure, your medical history and physical examination should describe characteristic symptoms and signs of pulmonary or systemic congestion or of limited cardiac output associated with the abnormal findings on appropriate medically acceptable imaging. When an acute episode of heart failure is triggered by a remediable factor, such as an arrhythmia, dietary sodium overload, or high altitude, cardiac function may be restored and a chronic impairment may not be present.
(i) Symptoms of congestion or of limited cardiac output include easy fatigue, weakness, shortness of breath (dyspnea), cough, or chest discomfort at rest or with activity. Individuals with CHF may also experience shortness of breath on lying flat (orthopnea) or episodes of shortness of breath that wake them from sleep (paroxysmal nocturnal dyspnea). They may also experience cardiac arrhythmias resulting in palpitations, lightheadedness, or fainting.
(ii) Signs of congestion may include hepatomegaly, ascites, increased jugular venous distention or pressure, rales, peripheral edema, or rapid weight gain. However, these signs need not be found on all examinations because fluid retention may be controlled by prescribed treatment.
3. Is it safe for you to have an ETT, if you have CHF? The presence of CHF is not necessarily a contraindication to an ETT, unless you are having an acute episode of heart failure. Measures of cardiac performance are valuable in helping us evaluate your ability to do work-related activities. Exercise testing has been safely used in individuals with CHF; therefore, we may purchase an ETT for evaluation under 4.02B3 if an MC, preferably one experienced in the care of patients with cardiovascular disease, determines that there is no significant risk to you. (See 4.00C6 for when we will consider the purchase of an ETT. See 4.00C7-4.00C8 for what we must do before we purchase an ETT and when we will not purchase one.) ST segment changes from digitalis use in the treatment of CHF do not preclude the purchase of an ETT.
4. How do we evaluate CHF using 4.02?
a. We must have objective evidence, as described in 4.00D2, that you have chronic heart failure.
b. To meet the required level of severity for this listing, your impairment must satisfy the requirements of one of the criteria in A and one of the criteria in B.
c. In 4.02B2, the phrase periods of stabilization means that, for at least 2 weeks between episodes of acute heart failure, there must be objective evidence of clearing of the pulmonary edema or pleural effusions and evidence that you returned to, or you were medically considered able to return to, your prior level of activity.
d. Listing 4.02B3c requires a decrease in systolic blood pressure below the baseline level (taken in the standing position immediately prior to exercise) or below any systolic pressure reading recorded during exercise. This is because, normally, systolic blood pressure and heart rate increase gradually with exercise. Decreases in systolic blood pressure below the baseline level that occur during exercise are often associated with ischemia-induced left ventricular dysfunction resulting in decreased cardiac output. However, a blunted response (that is, failure of the systolic blood pressure to rise 10 mm Hg or more), particularly in the first 3 minutes of exercise, may be drug-related and is not necessarily associated with left ventricular dysfunction. Also, some individuals with increased sympathetic responses because of deconditioning or apprehension may increase their systolic blood pressure and heart rate above their baseline level just before and early into exercise. This can be associated with a drop in systolic pressure in early exercise that is not due to left ventricular dysfunction. Therefore, an early decrease in systolic blood pressure must be interpreted within the total context of the test; that is, the presence or absence of symptoms such as lightheadedness, ischemic changes, or arrhythmias on the ECG.
E. Evaluating Ischemic Heart Disease1. What is ischemic heart disease (IHD)? IHD results when one or more of your coronary arteries is narrowed or obstructed or, in rare situations, constricted due to vasospasm, interfering with the normal flow of blood to your heart muscle (ischemia). The obstruction may be the result of an embolus, a thrombus, or plaque. When heart muscle tissue dies as a result of the reduced blood supply, it is called a myocardial infarction (heart attack).
2. What causes chest discomfort of myocardial origin?
a. Chest discomfort of myocardial ischemic origin, commonly known as angina pectoris, is usually caused by coronary artery disease (often abbreviated CAD). However, ischemic discomfort may be caused by a noncoronary artery impairment, such as aortic stenosis, hypertrophic cardiomyopathy, pulmonary hypertension, or anemia.
b. Instead of typical angina pectoris, some individuals with IHD experience atypical angina, anginal equivalent, variant angina, or silent ischemia, all of which we may evaluate using 4.04. We discuss the various manifestations of ischemia in 4.00E3-4.00E7.
3. What are the characteristics of typical angina pectoris? Discomfort of myocardial ischemic origin (angina pectoris) is discomfort that is precipitated by effort or emotion and promptly relieved by rest, sublingual nitroglycerin (that is, nitroglycerin tablets that are placed under the tongue), or other rapidly acting nitrates. Typically, the discomfort is located in the chest (usually substernal) and described as pressing, crushing, squeezing, burning, aching, or oppressive. Sharp, sticking, or cramping discomfort is less common. Discomfort occurring with activity or emotion should be described specifically as to timing and usual inciting factors (type and intensity), character, location, radiation, duration, and response to nitrate treatment or rest.
4. What is atypical angina? Atypical angina describes discomfort or pain from myocardial ischemia that is felt in places other than the chest. The common sites of cardiac pain are the inner aspect of the left arm, neck, jaw(s), upper abdomen, and back, but the discomfort or pain can be elsewhere. When pain of cardiac ischemic origin presents in an atypical site in the absence of chest discomfort, the source of the pain may be difficult to diagnose. To represent atypical angina, your discomfort or pain should have precipitating and relieving factors similar to those of typical chest discomfort, and we must have objective medical evidence of myocardial ischemia; for example, ECG or ETT evidence or appropriate medically acceptable imaging.
5. What is anginal equivalent? Often, individuals with IHD will complain of shortness of breath (dyspnea) on exertion without chest pain or discomfort. In a minority of such situations, the shortness of breath is due to myocardial ischemia; this is called anginal equivalent. To represent anginal equivalent, your shortness of breath should have precipitating and relieving factors similar to those of typical chest discomfort, and we must have objective medical evidence of myocardial ischemia; for example, ECG or ETT evidence or appropriate medically acceptable imaging. In these situations, it is essential to establish objective evidence of myocardial ischemia to ensure that you do not have effort dyspnea due to non-ischemic or non-cardiac causes.
6. What is variant angina?
a. Variant angina (Prinzmetal's angina, vasospastic angina) refers to the occurrence of anginal episodes at rest, especially at night, accompanied by transitory ST segment elevation (or, at times, ST depression) on an ECG. It is due to severe spasm of a coronary artery, causing ischemia of the heart wall, and is often accompanied by major ventricular arrhythmias, such as ventricular tachycardia. We will consider variant angina under 4.04 only if you have spasm of a coronary artery in relation to an obstructive lesion of the vessel. If you have an arrhythmia as a result of variant angina, we may consider your impairment under 4.05.
b. Variant angina may also occur in the absence of obstructive coronary disease. In this situation, an ETT will not demonstrate ischemia. The diagnosis will be established by showing the typical transitory ST segment changes during attacks of pain, and the absence of obstructive lesions shown by catheterization. Treatment in cases where there is no obstructive coronary disease is limited to medications that reduce coronary vasospasm, such as calcium channel blockers and nitrates. In such situations, we will consider the frequency of anginal episodes despite prescribed treatment when evaluating your residual functional capacity.
c. Vasospasm that is catheter-induced during coronary angiography is not variant angina.
7. What is silent ischemia?
a. Myocardial ischemia, and even myocardial infarction, can occur without perception of pain or any other symptoms; when this happens, we call it silent ischemia. Pain sensitivity may be altered by a variety of diseases, most notably diabetes mellitus and other neuropathic disorders. Individuals also vary in their threshold for pain.
b. Silent ischemia occurs most often in:
(i) Individuals with documented past myocardial infarction or established angina without prior infarction who do not have chest pain on ETT, but have a positive test with ischemic abnormality on ECG, perfusion scan, or other appropriate medically acceptable imaging.
(ii) Individuals with documented past myocardial infarction or angina who have ST segment changes on ambulatory monitoring (Holter monitoring) that are similar to those that occur during episodes of angina. ST depression shown on the ambulatory recording should not be interpreted as positive for ischemia unless similar depression is also seen during chest pain episodes annotated in the diary that the individual keeps while wearing the Holter monitor.
c. ST depression can result from a variety of factors, such as postural changes and variations in cardiac sympathetic tone. In addition, there are differences in how different Holter monitors record the electrical responses. Therefore, we do not consider the Holter monitor reliable for the diagnosis of silent ischemia except in the situation described in 4.00E7b(ii).
8. What other sources of chest discomfort are there? Chest discomfort of nonischemic origin may result from other cardiac impairments, such as pericarditis. Noncardiac impairments may also produce symptoms mimicking that of myocardial ischemia. These impairments include acute anxiety or panic attacks, gastrointestinal tract disorders, such as esophageal spasm, esophagitis, hiatal hernia, biliary tract disease, gastritis, peptic ulcer, and pancreatitis, and musculoskeletal syndromes, such as chest wall muscle spasm, chest wall syndrome (especially after coronary bypass surgery), costochondritis, and cervical or dorsal spine arthritis. Hyperventilation may also mimic ischemic discomfort. Thus, in the absence of documented myocardial ischemia, such disorders should be considered as possible causes of chest discomfort.
9. How do we evaluate IHD using 4.04?
a. We must have objective evidence, as described under 4.00C, that your symptoms are due to myocardial ischemia.
b. Listing-level changes on the ECG in 4.04A1 are the classically accepted changes of horizontal or downsloping ST depression occurring both during exercise and recovery. Although we recognize that ischemic changes may at times occur only during exercise or recovery, and may at times be upsloping with only junctional ST depression, such changes can be false positive; that is, occur in the absence of ischemia. Diagnosis of ischemia in this situation requires radionuclide or echocardiogram confirmation. See 4.00C12 and 4.00C13.
c. Also in 4.04A1, we require that the depression of the ST segment last for at least 1 minute of recovery because ST depression that occurs during exercise but that rapidly normalizes in recovery is a common false-positive response.
d. In 4.04A2, we specify that the ST elevation must be in non-infarct leads during both exercise and recovery. This is because, in the absence of ECG signs of prior infarction, ST elevation during exercise denotes ischemia, usually severe, requiring immediate termination of exercise. However, if there is baseline ST elevation in association with a prior infarction or ventricular aneurysm, further ST elevation during exercise does not necessarily denote ischemia and could be a false-positive ECG response. Diagnosis of ischemia in this situation requires radionuclide or echocardiogram confirmation. See 4.00C12 and 4.00C13.
e. Listing 4.04A3 requires a decrease in systolic blood pressure below the baseline level (taken in the standing position immediately prior to exercise) or below any systolic pressure reading recorded during exercise. This is the same finding required in 4.02B3c. See 4.00D4d for full details.
f. In 4.04B, each of the three ischemic episodes must require revascularization or be not amenable to treatment. Revascularization means angioplasty (with or without stent placement) or bypass surgery. However, reocclusion that occurs after a revascularization procedure but during the same hospitalization and that requires a second procedure during the same hospitalization will not be counted as another ischemic episode. Not amenable means that the revascularization procedure could not be done because of another medical impairment or because the vessel was not suitable for revascularization.
g. We will use 4.04C only when you have symptoms due to myocardial ischemia as described in 4.00E3-4.00E7 while on a regimen of prescribed treatment, you are at risk for exercise testing (see 4.00C8), and we do not have a timely ETT or a timely normal drug-induced stress test for you. See 4.00C9 for what we mean by a timely test.
h. In 4.04C1 the term nonbypassed means that the blockage is in a vessel that is potentially bypassable; that is, large enough to be bypassed and considered to be a cause of your ischemia. These vessels are usually major arteries or one of a major artery's major branches. A vessel that has become obstructed again after angioplasty or stent placement and has remained obstructed or is not amenable to another revascularization is considered a nonbypassed vessel for purposes of this listing. When you have had revascularization, we will not use the pre-operative findings to assess the current severity of your coronary artery disease under 4.04C, although we will consider the severity and duration of your impairment prior to your surgery in making our determination or decision.
F. Evaluating Arrhythmias1. What is an arrhythmia? An arrhythmia is a change in the regular beat of the heart. Your heart may seem to skip a beat or beat irregularly, very quickly (tachycardia), or very slowly (bradycardia).
2. What are the different types of arrhythmias?
a. There are many types of arrhythmias. Arrhythmias are identified by where they occur in the heart (atria or ventricles) and by what happens to the heart's rhythm when they occur.
b. Arrhythmias arising in the cardiac atria (upper chambers of the heart) are called atrial or supraventricular arrhythmias. Ventricular arrhythmias begin in the ventricles (lower chambers). In general, ventricular arrhythmias caused by heart disease are the most serious.
3. How do we evaluate arrhythmias using 4.05?
a. We will use 4.05 when you have arrhythmias that are not fully controlled by medication, an implanted pacemaker, or an implanted cardiac defibrillator and you have uncontrolled recurrent episodes of syncope or near syncope. If your arrhythmias are controlled, we will evaluate your underlying heart disease using the appropriate listing. For other considerations when we evaluate arrhythmias in the presence of an implanted cardiac defibrillator, see 4.00F4.
b. We consider near syncope to be a period of altered consciousness, since syncope is a loss of consciousness or a faint. It is not merely a feeling of light-headedness, momentary weakness, or dizziness.
c. For purposes of 4.05, there must be a documented association between the syncope or near syncope and the recurrent arrhythmia. The recurrent arrhythmia, not some other cardiac or non-cardiac disorder, must be established as the cause of the associated symptom. This documentation of the association between the symptoms and the arrhythmia may come from the usual diagnostic methods, including Holter monitoring (also called ambulatory electrocardiography) and tilt-table testing with a concurrent ECG. Although an arrhythmia may be a coincidental finding on an ETT, we will not purchase an ETT to document the presence of a cardiac arrhythmia.
4. What will we consider when you have an implanted cardiac defibrillator and you do not have arrhythmias that meet the requirements of 4.05?
a. Implanted cardiac defibrillators are used to prevent sudden cardiac death in individuals who have had, or are at high risk for, cardiac arrest from life-threatening ventricular arrhythmias. The largest group at risk for sudden cardiac death consists of individuals with cardiomyopathy (ischemic or non-ischemic) and reduced ventricular function. However, life-threatening ventricular arrhythmias can also occur in individuals with little or no ventricular dysfunction. The shock from the implanted cardiac defibrillator is a unique form of treatment; it rescues an individual from what may have been cardiac arrest. However, as a consequence of the shock(s), individuals may experience psychological distress, which we may evaluate under the mental disorders listings in 12.00ff.
b. Most implantable cardiac defibrillators have rhythm-correcting and pacemaker capabilities. In some individuals, these functions may result in the termination of ventricular arrhythmias without an otherwise painful shock. (The shock is like being kicked in the chest.) Implanted cardiac defibrillators may deliver inappropriate shocks, often repeatedly, in response to benign arrhythmias or electrical malfunction. Also, exposure to strong electrical or magnetic fields, such as from MRI (magnetic resonance imaging), can trigger or reprogram an implanted cardiac defibrillator, resulting in inappropriate shocks. We must consider the frequency of, and the reason(s) for, the shocks when evaluating the severity and duration of your impairment.
c. In general, the exercise limitations imposed on individuals with an implanted cardiac defibrillator are those dictated by the underlying heart impairment. However, the exercise limitations may be greater when the implanted cardiac defibrillator delivers an inappropriate shock in response to the increase in heart rate with exercise, or when there is exercise-induced ventricular arrhythmia.
G. Evaluating Peripheral Vascular Disease1. What is peripheral vascular disease (PVD)? Generally, PVD is any impairment that affects either the arteries (peripheral arterial disease) or the veins (venous insufficiency) in the extremities, particularly the lower extremities. The usual effect is blockage of the flow of blood either from the heart (arterial) or back to the heart (venous). If you have peripheral arterial disease, you may have pain in your calf after walking a distance that goes away when you rest (intermittent claudication); at more advanced stages, you may have pain in your calf at rest or you may develop ulceration or gangrene. If you have venous insufficiency, you may have swelling, varicose veins, skin pigmentation changes, or skin ulceration.
2. How do we assess limitations resulting from PVD? We will assess your limitations based on your symptoms together with physical findings, Doppler studies, other appropriate non-invasive studies, or angiographic findings. However, if the PVD has resulted in amputation, we will evaluate any limitations related to the amputation under the musculoskeletal listings, 1.00ff.
3. What is brawny edema? Brawny edema (4.11A) is swelling that is usually dense and feels firm due to the presence of increased connective tissue; it is also associated with characteristic skin pigmentation changes. It is not the same thing as pitting edema. Brawny edema generally does not pit (indent on pressure), and the terms are not interchangeable. Pitting edema does not satisfy the requirements of 4.11A.
4. What is lymphedema and how will we evaluate it?
a. Lymphedema is edema of the extremities due to a disorder of the lymphatic circulation; at its worst, it is called elephantiasis. Primary lymphedema is caused by abnormal development of lymph vessels and may be present at birth (congenital lymphedema), but more often develops during the teens (lymphedema praecox). It may also appear later, usually after age 35 (lymphedema tarda). Secondary lymphedema is due to obstruction or destruction of normal lymphatic channels due to tumor, surgery, repeated infections, or parasitic infection such as filariasis. Lymphedema most commonly affects one extremity.
b. Lymphedema does not meet the requirements of 4.11, although it may medically equal the severity of that listing. We will evaluate lymphedema by considering whether the underlying cause meets or medically equals any listing or whether the lymphedema medically equals a cardiovascular listing, such as 4.11, or a musculoskeletal listing, such as 1.02A or 1.03. If no listing is met or medically equaled, we will evaluate any functional limitations imposed by your lymphedema when we assess your residual functional capacity.
5. When will we purchase exercise Doppler studies for evaluating peripheral arterial disease (PAD)? If we need additional evidence of your PAD, we will generally purchase exercise Doppler studies (see 4.00C16 and 4.00C17) when your resting ankle/brachial systolic blood pressure ratio is at least 0.50 but less than 0.80, and only rarely when it is 0.80 or above. We will not purchase exercise Doppler testing if you have a disease that results in abnormal arterial calcification or small vessel disease, but will use your resting toe systolic blood pressure or resting toe/brachial systolic blood pressure ratio. (See 4.00G7c and 4.00G8.) There are no current medical standards for evaluating exercise toe pressures. Because any exercise test stresses your entire cardiovascular system, we will purchase exercise Doppler studies only after an MC, preferably one with experience in the care of patients with cardiovascular disease, has determined that the test would not present a significant risk to you and that there is no other medical reason not to purchase the test (see 4.00C6, 4.00C7, and 4.00C8).
6. Are there any other studies that are helpful in evaluating PAD? Doppler studies done using a recording ultrasonic Doppler unit and strain-gauge plethysmography are other useful tools for evaluating PAD. A recording Doppler, which prints a tracing of the arterial pulse wave in the femoral, popliteal, dorsalis pedis, and posterior tibial arteries, is an excellent evaluation tool to compare wave forms in normal and compromised peripheral blood flow. Qualitative analysis of the pulse wave is very helpful in the overall assessment of the severity of the occlusive disease. Tracings are especially helpful in assessing severity if you have small vessel disease related to diabetes mellitus or other diseases with similar vascular changes, or diseases causing medial calcifications when ankle pressure is either normal or falsely high.
7. How do we evaluate PAD under 4.12?
a. The ankle blood pressure referred to in 4.12A and B is the higher of the pressures recorded from the posterior tibial and dorsalis pedis arteries in the affected leg. The higher pressure recorded from the two sites is the more significant measurement in assessing the extent of arterial insufficiency. Techniques for obtaining ankle systolic blood pressures include Doppler (See 4.00C16 and 4.00C17), plethysmographic studies, or other techniques. We will request any available tracings generated by these studies so that we can review them.
b. In 4.12A, the ankle/brachial systolic blood pressure ratio is the ratio of the systolic blood pressure at the ankle to the systolic blood pressure at the brachial artery; both taken at the same time while you are lying on your back. We do not require that the ankle and brachial pressures be taken on the same side of your body. This is because, as with the ankle pressure, we will use the higher brachial systolic pressure measured. Listing 4.12A is met when your resting ankle/brachial systolic blood pressure ratio is less than 0.50. If your resting ankle/brachial systolic blood pressure ratio is 0.50 or above, we will use 4.12B to evaluate the severity of your PAD, unless you also have a disease causing abnormal arterial calcification or small vessel disease, such as diabetes mellitus. See 4.00G7c and 4.00G8.
c. We will use resting toe systolic blood pressures or resting toe/brachial systolic blood pressure ratios (determined the same way as ankle/brachial ratios, see 4.00G7b) when you have intermittent claudication and a disease that results in abnormal arterial calcification (for example, Monckeberg's sclerosis or diabetes mellitus) or small vessel disease (for example, diabetes mellitus). These diseases may result in misleadingly high blood pressure readings at the ankle. However, high blood pressures due to vascular changes related to these diseases seldom occur at the toe level. While the criteria in 4.12C and 4.12D are intended primarily for individuals who have a disease causing abnormal arterial calcification or small vessel disease, we may also use them for evaluating anyone with PAD.
8. How are toe pressures measured? Toe pressures are measured routinely in most vascular laboratories through one of three methods: most frequently, photoplethysmography; less frequently, plethysmography using strain gauge cuffs; and Doppler ultrasound. Toe pressure can also be measured by using any blood pressure cuff that fits snugly around the big toe and is neither too tight nor too loose. A neonatal cuff or a cuff designed for use on fingers or toes can be used in the measurement of toe pressure.
9. How do we use listing 4.12 if you have had a peripheral graft? Peripheral grafting serves the same purpose as coronary grafting; that is, to bypass a narrow or obstructed arterial segment. If intermittent claudication recurs or persists after peripheral grafting, we may purchase Doppler studies to assess the flow of blood through the bypassed vessel and to establish the current severity of the peripheral arterial impairment. However, if you have had peripheral grafting done for your PAD, we will not use the findings from before the surgery to assess the current severity of your impairment, although we will consider the severity and duration of your impairment prior to your surgery in making our determination or decision.
H. Evaluating Other Cardiovascular Impairments1. How will we evaluate hypertension? Because hypertension (high blood pressure) generally causes disability through its effects on other body systems, we will evaluate it by reference to the specific body system(s) affected (heart, brain, kidneys, or eyes) when we consider its effects under the listings. We will also consider any limitations imposed by your hypertension when we assess your residual functional capacity.
2. How will we evaluate symptomatic congenital heart disease? Congenital heart disease is any abnormality of the heart or the major blood vessels that is present at birth. Because of improved treatment methods, more children with congenital heart disease are living to adulthood. Although some types of congenital heart disease may be corrected by surgery, many individuals with treated congenital heart disease continue to have problems throughout their lives (symptomatic congenital heart disease). If you have congenital heart disease that results in chronic heart failure with evidence of ventricular dysfunction or in recurrent arrhythmias, we will evaluate your impairment under 4.02 or 4.05. Otherwise, we will evaluate your impairment under 4.06.
3. What is cardiomyopathy and how will we evaluate it? Cardiomyopathy is a disease of the heart muscle. The heart loses its ability to pump blood (heart failure), and in some instances, heart rhythm is disturbed, leading to irregular heartbeats (arrhythmias). Usually, the exact cause of the muscle damage is never found (idiopathic cardiomyopathy). There are various types of cardiomyopathy, which fall into two major categories: Ischemic and nonischemic cardiomyopathy. Ischemic cardiomyopathy typically refers to heart muscle damage that results from coronary artery disease, including heart attacks. Nonischemic cardiomyopathy includes several types: Dilated, hypertrophic, and restrictive. We will evaluate cardiomyopathy under 4.02, 4.04, 4.05, or 11.04, depending on its effects on you.
4. How will we evaluate valvular heart disease? We will evaluate valvular heart disease under the listing appropriate for its effect on you. Thus, we may use 4.02, 4.04, 4.05, 4.06, or an appropriate neurological listing in 11.00ff.
5. What do we consider when we evaluate heart transplant recipients?
a. After your heart transplant, we will consider you disabled for 1 year following the surgery because there is a greater likelihood of rejection of the organ and infection during the first year.
b. However, heart transplant patients generally meet our definition of disability before they undergo transplantation. We will determine the onset of your disability based on the facts in your case.
c. We will not assume that you became disabled when your name was placed on a transplant waiting list. This is because you may be placed on a waiting list soon after diagnosis of the cardiac disorder that may eventually require a transplant. Physicians recognize that candidates for transplantation often have to wait months or even years before a suitable donor heart is found, so they place their patients on the list as soon as permitted.
d. When we do a continuing disability review to determine whether you are still disabled, we will evaluate your residual impairment(s), as shown by symptoms, signs, and laboratory findings, including any side effects of medication. We will consider any remaining symptoms, signs, and laboratory findings indicative of cardiac dysfunction in deciding whether medical improvement (as defined in §§ 404.1594 and 416.994) has occurred.
6. When does an aneurysm have “dissection not controlled by prescribed treatment,” as required under 4.10? An aneurysm (or bulge in the aorta or one of its major branches) is dissecting when the inner lining of the artery begins to separate from the arterial wall. We consider the dissection not controlled when you have persistence of chest pain due to progression of the dissection, an increase in the size of the aneurysm, or compression of one or more branches of the aorta supplying the heart, kidneys, brain, or other organs. An aneurysm with dissection can cause heart failure, renal (kidney) failure, or neurological complications. If you have an aneurysm that does not meet the requirements of 4.10 and you have one or more of these associated conditions, we will evaluate the condition(s) using the appropriate listing.
7. What is hyperlipidemia and how will we evaluate it? Hyperlipidemia is the general term for an elevation of any or all of the lipids (fats or cholesterol) in the blood; for example, hypertriglyceridemia, hypercholesterolemia, and hyperlipoproteinemia. These disorders of lipoprotein metabolism and transport can cause defects throughout the body. The effects most likely to interfere with function are those produced by atherosclerosis (narrowing of the arteries) and coronary artery disease. We will evaluate your lipoprotein disorder by considering its effects on you.
8. What is Marfan syndrome and how will we evaluate it?
a. Marfan syndrome is a genetic connective tissue disorder that affects multiple body systems, including the skeleton, eyes, heart, blood vessels, nervous system, skin, and lungs. There is no specific laboratory test to diagnose Marfan syndrome. The diagnosis is generally made by medical history, including family history, physical examination, including an evaluation of the ratio of arm/leg size to trunk size, a slit lamp eye examination, and a heart test(s), such as an echocardiogram. In some cases, a genetic analysis may be useful, but such analyses may not provide any additional helpful information.
b. The effects of Marfan syndrome can range from mild to severe. In most cases, the disorder progresses as you age. Most individuals with Marfan syndrome have abnormalities associated with the heart and blood vessels. Your heart's mitral valve may leak, causing a heart murmur. Small leaks may not cause symptoms, but larger ones may cause shortness of breath, fatigue, and palpitations. Another effect is that the wall of the aorta may be weakened and abnormally stretch (aortic dilation). This aortic dilation may tear, dissect, or rupture, causing serious heart problems or sometimes sudden death. We will evaluate the manifestations of your Marfan syndrome under the appropriate body system criteria, such as 4.10, or if necessary, consider the functional limitations imposed by your impairment.
I. Other Evaluation Issues1. What effect does obesity have on the cardiovascular system and how will we evaluate it? Obesity is a medically determinable impairment that is often associated with disorders of the cardiovascular system. Disturbance of this system can be a major cause of disability if you have obesity. Obesity may affect the cardiovascular system because of the increased workload the additional body mass places on the heart. Obesity may make it harder for the chest and lungs to expand. This can mean that the respiratory system must work harder to provide needed oxygen. This in turn would make the heart work harder to pump blood to carry oxygen to the body. Because the body would be working harder at rest, its ability to perform additional work would be less than would otherwise be expected. Thus, the combined effects of obesity with cardiovascular impairments can be greater than the effects of each of the impairments considered separately. We must consider any additional and cumulative effects of obesity when we determine whether you have a severe cardiovascular impairment or a listing-level cardiovascular impairment (or a combination of impairments that medically equals the severity of a listed impairment), and when we assess your residual functional capacity.
2. How do we relate treatment to functional status? In general, conclusions about the severity of a cardiovascular impairment cannot be made on the basis of type of treatment rendered or anticipated. The amount of function restored and the time required for improvement after treatment (medical, surgical, or a prescribed program of progressive physical activity) vary with the nature and extent of the disorder, the type of treatment, and other factors. Depending upon the timing of this treatment in relation to the alleged onset date of disability, we may need to defer evaluation of the impairment for a period of up to 3 months from the date treatment began to permit consideration of treatment effects, unless we can make a determination or decision using the evidence we have. See 4.00B4.
3. How do we evaluate impairments that do not meet one of the cardiovascular listings?
a. These listings are only examples of common cardiovascular impairments that we consider severe enough to prevent you from doing any gainful activity. If your severe impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
b. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairments(s) medically equals a listing. (See §§ 404.1526 and 416.926.) If you have a severe impairment(s) that does not meet or medically equal the criteria of a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. Therefore, we proceed to the fourth and, if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920. If you are an adult, we use the rules in §§ 404.1594 or 416.994, as appropriate, when we decide whether you continue to be disabled.
4.01 Category of Impairments, Cardiovascular System4.02 Chronic heart failure while on a regimen of prescribed treatment, with symptoms and signs described in 4.00D2. The required level of severity for this impairment is met when the requirements in both A and B are satisfied.
A. Medically documented presence of one of the following:
1. Systolic failure (see 4.00D1a(i)), with left ventricular end diastolic dimensions greater than 6.0 cm or ejection fraction of 30 percent or less during a period of stability (not during an episode of acute heart failure); or
2. Diastolic failure (see 4.00D1a(ii)), with left ventricular posterior wall plus septal thickness totaling 2.5 cm or greater on imaging, with an enlarged left atrium greater than or equal to 4.5 cm, with normal or elevated ejection fraction during a period of stability (not during an episode of acute heart failure);
ANDB. Resulting in one of the following:
1. Persistent symptoms of heart failure which very seriously limit the ability to independently initiate, sustain, or complete activities of daily living in an individual for whom an MC, preferably one experienced in the care of patients with cardiovascular disease, has concluded that the performance of an exercise test would present a significant risk to the individual; or
2. Three or more separate episodes of acute congestive heart failure within a consecutive 12-month period (see 4.00A3e), with evidence of fluid retention (see 4.00D2b(ii)) from clinical and imaging assessments at the time of the episodes, requiring acute extended physician intervention such as hospitalization or emergency room treatment for 12 hours or more, separated by periods of stabilization (see 4.00D4c); or
3. Inability to perform on an exercise tolerance test at a workload equivalent to 5 METs or less due to:
a. Dyspnea, fatigue, palpitations, or chest discomfort; or
b. Three or more consecutive premature ventricular contractions (ventricular tachycardia), or increasing frequency of ventricular ectopy with at least 6 premature ventricular contractions per minute; or
c. Decrease of 10 mm Hg or more in systolic pressure below the baseline systolic blood pressure or the preceding systolic pressure measured during exercise (see 4.00D4d) due to left ventricular dysfunction, despite an increase in workload; or
d. Signs attributable to inadequate cerebral perfusion, such as ataxic gait or mental confusion.
4.04 Ischemic heart disease, with symptoms due to myocardial ischemia, as described in 4.00E3-4.00E7, while on a regimen of prescribed treatment (see 4.00B3 if there is no regimen of prescribed treatment), with one of the following:
A. Sign-or symptom-limited exercise tolerance test demonstrating at least one of the following manifestations at a workload equivalent to 5 METs or less:
1. Horizontal or downsloping depression, in the absence of digitalis glycoside treatment or hypokalemia, of the ST segment of at least −0.10 millivolts (−1.0 mm) in at least 3 consecutive complexes that are on a level baseline in any lead other than aVR, and depression of at least −0.10 millivolts lasting for at least 1 minute of recovery; or
2. At least 0.1 millivolt (1 mm) ST elevation above resting baseline in non-infarct leads during both exercise and 1 or more minutes of recovery; or
3. Decrease of 10 mm Hg or more in systolic pressure below the baseline blood pressure or the preceding systolic pressure measured during exercise (see 4.00E9e) due to left ventricular dysfunction, despite an increase in workload; or
4. Documented ischemia at an exercise level equivalent to 5 METs or less on appropriate medically acceptable imaging, such as radionuclide perfusion scans or stress echocardiography.
ORB. Three separate ischemic episodes, each requiring revascularization or not amenable to revascularization (see 4.00E9f), within a consecutive 12-month period (see 4.00A3e).
ORC. Coronary artery disease, demonstrated by angiography (obtained independent of Social Security disability evaluation) or other appropriate medically acceptable imaging, and in the absence of a timely exercise tolerance test or a timely normal drug-induced stress test, an MC, preferably one experienced in the care of patients with cardiovascular disease, has concluded that performance of exercise tolerance testing would present a significant risk to the individual, with both 1 and 2:
1. Angiographic evidence showing:
a. 50 percent or more narrowing of a nonbypassed left main coronary artery; or
b. 70 percent or more narrowing of another nonbypassed coronary artery; or
c. 50 percent or more narrowing involving a long (greater than 1 cm) segment of a nonbypassed coronary artery; or
d. 50 percent or more narrowing of at least two nonbypassed coronary arteries; or
e. 70 percent or more narrowing of a bypass graft vessel; and
2. Resulting in very serious limitations in the ability to independently initiate, sustain, or complete activities of daily living.
4.05 Recurrent arrhythmias, not related to reversible causes, such as electrolyte abnormalities or digitalis glycoside or antiarrhythmic drug toxicity, resulting in uncontrolled (see 4.00A3f), recurrent (see 4.00A3c) episodes of cardiac syncope or near syncope (see 4.00F3b), despite prescribed treatment (see 4.00B3 if there is no prescribed treatment), and documented by resting or ambulatory (Holter) electrocardiography, or by other appropriate medically acceptable testing, coincident with the occurrence of syncope or near syncope (see 4.00F3c).
4.06 Symptomatic congenital heart disease (cyanotic or acyanotic), documented by appropriate medically acceptable imaging (see 4.00A3d) or cardiac catheterization, with one of the following:
A. Cyanosis at rest, and:
1. Hematocrit of 55 percent or greater; or
2. Arterial O2 saturation of less than 90 percent in room air, or resting arterial PO2 of 60 Torr or less.
ORB. Intermittent right-to-left shunting resulting in cyanosis on exertion (e.g., Eisenmenger's physiology) and with arterial PO2 of 60 Torr or less at a workload equivalent to 5 METs or less.
ORC. Secondary pulmonary vascular obstructive disease with pulmonary arterial systolic pressure elevated to at least 70 percent of the systemic arterial systolic pressure.
4.09 Heart transplant. Consider under a disability for 1 year following surgery; thereafter, evaluate residual impairment under the appropriate listing.
4.10 Aneurysm of aorta or major branches, due to any cause (e.g., atherosclerosis, cystic medial necrosis, Marfan syndrome, trauma), demonstrated by appropriate medically acceptable imaging, with dissection not controlled by prescribed treatment (see 4.00H6).
4.11 Chronic venous insufficiency of a lower extremity with incompetency or obstruction of the deep venous system and one of the following:
A. Extensive brawny edema (see 4.00G3) involving at least two-thirds of the leg between the ankle and knee or the distal one-third of the lower extremity between the ankle and hip.
ORB. Superficial varicosities, stasis dermatitis, and either recurrent ulceration or persistent ulceration that has not healed following at least 3 months of prescribed treatment.
4.12 Peripheral arterial disease, as determined by appropriate medically acceptable imaging (see 4.00A3d, 4.00G2, 4.00G5, and 4.00G6), causing intermittent claudication (see 4.00G1) and one of the following:
A. Resting ankle/brachial systolic blood pressure ratio of less than 0.50.
ORB. Decrease in systolic blood pressure at the ankle on exercise (see 4.00G7a and 4.00C16-4.00C17) of 50 percent or more of pre-exercise level and requiring 10 minutes or more to return to pre-exercise level.
ORC. Resting toe systolic pressure of less than 30 mm Hg (see 4.00G7c and 4.00G8).
ORD. Resting toe/brachial systolic blood pressure ratio of less than 0.40 (see 4.00G7c).
5.00 Digestive SystemA. What kinds of disorders do we consider in the digestive system? Disorders of the digestive system include gastrointestinal hemorrhage, hepatic (liver) dysfunction, inflammatory bowel disease, short bowel syndrome, and malnutrition. They may also lead to complications, such as obstruction, or be accompanied by manifestations in other body systems.
B. What documentation do we need? We need a record of your medical evidence, including clinical and laboratory findings. The documentation should include appropriate medically acceptable imaging studies and reports of endoscopy, operations, and pathology, as appropriate to each listing, to document the severity and duration of your digestive disorder. Medically acceptable imaging includes, but is not limited to, x-ray imaging, sonography, computerized axial tomography (CAT scan), magnetic resonance imaging (MRI), and radionuclide scans. Appropriate means that the technique used is the proper one to support the evaluation and diagnosis of the disorder. The findings required by these listings must occur within the period we are considering in connection with your application or continuing disability review.
C. How do we consider the effects of treatment?
1. Digestive disorders frequently respond to medical or surgical treatment; therefore, we generally consider the severity and duration of these disorders within the context of prescribed treatment.
2. We assess the effects of treatment, including medication, therapy, surgery, or any other form of treatment you receive, by determining if there are improvements in the symptoms, signs, and laboratory findings of your digestive disorder. We also assess any side effects of your treatment that may further limit your functioning.
3. To assess the effects of your treatment, we may need information about:
a. The treatment you have been prescribed (for example, the type of medication or therapy, or your use of parenteral (intravenous) nutrition or supplemental enteral nutrition via a gastrostomy);
b. The dosage, method, and frequency of administration;
c. Your response to the treatment;
d. Any adverse effects of such treatment; and
e. The expected duration of the treatment.
4. Because the effects of treatment may be temporary or long-term, in most cases we need information about the impact of your treatment, including its expected duration and side effects, over a sufficient period of time to help us assess its outcome. When adverse effects of treatment contribute to the severity of your impairment(s), we will consider the duration or expected duration of the treatment when we assess the duration of your impairment(s).
5. If you need parenteral (intravenous) nutrition or supplemental enteral nutrition via a gastrostomy to avoid debilitating complications of a digestive disorder, this treatment will not, in itself, indicate that you are unable to do any gainful activity, except under 5.07, short bowel syndrome (see 5.00F).
6. If you have not received ongoing treatment or have not had an ongoing relationship with the medical community despite the existence of a severe impairment(s), we will evaluate the severity and duration of your digestive impairment on the basis of the current medical and other evidence in your case record. If you have not received treatment, you may not be able to show an impairment that meets the criteria of one of the digestive system listings, but your digestive impairment may medically equal a listing or be disabling based on consideration of your residual functional capacity, age, education, and work experience.
D. How do we evaluate chronic liver disease?
1. General. Chronic liver disease is characterized by liver cell necrosis, inflammation, or scarring (fibrosis or cirrhosis), due to any cause, that persists for more than 6 months. Chronic liver disease may result in portal hypertension, cholestasis (suppression of bile flow), extrahepatic manifestations, or liver cancer. (We evaluate liver cancer under 13.19.) Significant loss of liver function may be manifested by hemorrhage from varices or portal hypertensive gastropathy, ascites (accumulation of fluid in the abdominal cavity), hydrothorax (ascitic fluid in the chest cavity), or encephalopathy. There can also be progressive deterioration of laboratory findings that are indicative of liver dysfunction. Liver transplantation is the only definitive cure for end stage liver disease (ESLD).
2. Examples of chronic liver disease include, but are not limited to, chronic hepatitis, alcoholic liver disease, non-alcoholic steatohepatitis (NASH), primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), autoimmune hepatitis, hemochromatosis, drug-induced liver disease, Wilson's disease, and serum alpha-1 antitrypsin deficiency. Acute hepatic injury is frequently reversible, as in viral, drug-induced, toxin-induced, alcoholic, and ischemic hepatitis. In the absence of evidence of a chronic impairment, episodes of acute liver disease do not meet 5.05.
3. Manifestations of chronic liver disease.
a. Symptoms may include, but are not limited to, pruritis (itching), fatigue, nausea, loss of appetite, or sleep disturbances. Symptoms of chronic liver disease may have a poor correlation with the severity of liver disease and functional ability.
b. Signs may include, but are not limited to, jaundice, enlargement of the liver and spleen, ascites, peripheral edema, and altered mental status.
c. Laboratory findings may include, but are not limited to, increased liver enzymes, increased serum total bilirubin, increased ammonia levels, decreased serum albumin, and abnormal coagulation studies, such as increased International Normalized Ratio (INR) or decreased platelet counts. Abnormally low serum albumin or elevated INR levels indicate loss of synthetic liver function, with increased likelihood of cirrhosis and associated complications. However, other abnormal lab tests, such as liver enzymes, serum total bilirubin, or ammonia levels, may have a poor correlation with the severity of liver disease and functional ability. A liver biopsy may demonstrate the degree of liver cell necrosis, inflammation, fibrosis, and cirrhosis. If you have had a liver biopsy, we will make every reasonable effort to obtain the results; however, we will not purchase a liver biopsy. Imaging studies (CAT scan, ultrasound, MRI) may show the size and consistency (fatty liver, scarring) of the liver and document ascites (see 5.00D6).
4. Chronic viral hepatitis infections.
a. General.
(i) Chronic viral hepatitis infections are commonly caused by hepatitis C virus (HCV), and to a lesser extent, hepatitis B virus (HBV). Usually, these are slowly progressive disorders that persist over many years during which the symptoms and signs are typically nonspecific, intermittent, and mild (for example, fatigue, difficulty with concentration, or right upper quadrant pain). Laboratory findings (liver enzymes, imaging studies, liver biopsy pathology) and complications are generally similar in HCV and HBV. The spectrum of these chronic viral hepatitis infections ranges widely and includes an asymptomatic state; insidious disease with mild to moderate symptoms associated with fluctuating liver tests; extrahepatic manifestations; cirrhosis, both compensated and decompensated; ESLD with the need for liver transplantation; and liver cancer. Treatment for chronic viral hepatitis infections varies considerably based on medication tolerance, treatment response, adverse effects of treatment, and duration of the treatment. Comorbid disorders, such as HIV infection, may accelerate the clinical course of viral hepatitis infection(s) or may result in a poorer response to medical treatment.
(ii) We evaluate all types of chronic viral hepatitis infections under 5.05 or any listing in an affected body system(s). If your impairment(s) does not meet or medically equal a listing, we will consider the effects of your hepatitis when we assess your residual functional capacity.
b. Chronic hepatitis B virus (HBV) infection.
(i) Chronic HBV infection can be diagnosed by the detection of hepatitis B surface antigen (HBsAg) or hepatitis B virus DNA (HBV DNA) in the blood for at least 6 months. In addition, detection of the hepatitis B e antigen (HBeAg) suggests an increased likelihood of progression to cirrhosis, ESLD, and hepatocellular carcinoma. (HBeAg may also be referred to as “hepatitis B early antigen” or “hepatitis B envelope antigen.”)
(ii) The therapeutic goal of treatment is to suppress HBV replication and thereby prevent progression to cirrhosis, ESLD, and hepatocellular carcinoma. Treatment usually includes interferon injections, oral antiviral agents, or a combination of both. Common adverse effects of treatment are the same as noted in 5.00D4c(ii) for HCV, and generally end within a few days after treatment is discontinued.
c. Chronic hepatitis C virus (HCV) infection.
(i) Chronic HCV infection is diagnosed by the detection of hepatitis C viral RNA in the blood for at least 6 months. Documentation of the therapeutic response to treatment is also monitored by the quantitative assay of serum HCV RNA (“HCV viral load”). Treatment usually includes a combination of interferon injections and oral ribavirin; whether a therapeutic response has occurred is usually assessed after 12 weeks of treatment by checking the HCV viral load. If there has been a substantial reduction in HCV viral load (also known as early viral response, or EVR), this reduction is predictive of a sustained viral response with completion of treatment. Combined therapy is commonly discontinued after 12 weeks when there is no early viral response, since in that circumstance there is little chance of obtaining a sustained viral response (SVR). Otherwise, treatment is usually continued for a total of 48 weeks.
(ii) Combined interferon and ribavirin treatment may have significant adverse effects that may require dosing reduction, planned interruption of treatment, or discontinuation of treatment. Adverse effects may include: Anemia (ribavirin-induced hemolysis), neutropenia, thrombocytopenia, fever, cough, fatigue, myalgia, arthralgia, nausea, loss of appetite, pruritis, and insomnia. Behavioral side effects may also occur. Influenza-like symptoms are generally worse in the first 4 to 6 hours after each interferon injection and during the first weeks of treatment. Adverse effects generally end within a few days after treatment is discontinued.
d. Extrahepatic manifestations of HBV and HCV. In addition to their hepatic manifestations, both HBV and HCV may have significant extrahepatic manifestations in a variety of body systems. These include, but are not limited to: Keratoconjunctivitis (sicca syndrome), glomerulonephritis, skin disorders (for example, lichen planus, porphyria cutanea tarda), neuropathy, and immune dysfunction (for example, cryoglobulinemia, Sjögren's syndrome, and vasculitis). The extrahepatic manifestations of HBV and HCV may not correlate with the severity of your hepatic impairment. If your impairment(s) does not meet or medically equal a listing in an affected body system(s), we will consider the effects of your extrahepatic manifestations when we assess your residual functional capacity.
5. Gastrointestinal hemorrhage (5.02 and 5.05A). Gastrointestinal hemorrhaging can result in hematemesis (vomiting of blood), melena (tarry stools), or hematochezia (bloody stools). Under 5.02, the required transfusions of at least 2 units of blood must be at least 30 days apart and occur at least three times during a consecutive 6-month period. Under 5.05A, hemodynamic instability is diagnosed with signs such as pallor (pale skin), diaphoresis (profuse perspiration), rapid pulse, low blood pressure, postural hypotension (pronounced fall in blood pressure when arising to an upright position from lying down) or syncope (fainting). Hemorrhaging that results in hemodynamic instability is potentially life-threatening and therefore requires hospitalization for transfusion and supportive care. Under 5.05A, we require only one hospitalization for transfusion of at least 2 units of blood.
6. Ascites or hydrothorax (5.05B) indicates significant loss of liver function due to chronic liver disease. We evaluate ascites or hydrothorax that is not attributable to other causes under 5.05B. The required findings must be present on at least two evaluations at least 60 days apart within a consecutive 6-month period and despite continuing treatment as prescribed.
7. Spontaneous bacterial peritonitis (5.05C) is an infectious complication of chronic liver disease. It is diagnosed by ascitic peritoneal fluid that is documented to contain an absolute neutrophil count of at least 250 cells/mm 3. The required finding in 5.05C is satisfied with one evaluation documenting peritoneal fluid infection. We do not evaluate other causes of peritonitis that are unrelated to chronic liver disease, such as tuberculosis, malignancy, and perforated bowel, under this listing. We evaluate these other causes of peritonitis under the appropriate body system listings.
8. Hepatorenal syndrome (5.05D) is defined as functional renal failure associated with chronic liver disease in the absence of underlying kidney pathology. Hepatorenal syndrome is documented by elevation of serum creatinine, marked sodium retention, and oliguria (reduced urine output). The requirements of 5.05D are satisfied with documentation of any one of the three laboratory findings on one evaluation. We do not evaluate known causes of renal dysfunction, such as glomerulonephritis, tubular necrosis, drug-induced renal disease, and renal infections, under this listing. We evaluate these other renal impairments under 6.00ff.
9. Hepatopulmonary syndrome (5.05E) is defined as arterial deoxygenation (hypoxemia) that is associated with chronic liver disease due to intrapulmonary arteriovenous shunting and vasodilatation in the absence of other causes of arterial deoxygenation. Clinical manifestations usually include dyspnea, orthodeoxia (increasing hypoxemia with erect position), platypnea (improvement of dyspnea with flat position), cyanosis, and clubbing. The requirements of 5.05E are satisfied with documentation of any one of the findings on one evaluation. In 5.05E1, we require documentation of the altitude of the testing facility because altitude affects the measurement of arterial oxygenation. We will not purchase the specialized studies described in 5.05E2; however, if you have had these studies at a time relevant to your claim, we will make every reasonable effort to obtain the reports for the purpose of establishing whether your impairment meets 5.05E2.
10. Hepatic encephalopathy (5.05F).
a. General. Hepatic encephalopathy usually indicates severe loss of hepatocellular function. We define hepatic encephalopathy under 5.05F as a recurrent or chronic neuropsychiatric disorder, characterized by abnormal behavior, cognitive dysfunction, altered state of consciousness, and ultimately coma and death. The diagnosis is established by changes in mental status associated with fleeting neurological signs, including “flapping tremor” (asterixis), characteristic electroencephalographic (EEG) abnormalities, or abnormal laboratory values that indicate loss of synthetic liver function. We will not purchase the EEG testing described in 5.05F3b; however, if you have had this test at a time relevant to your claim, we will make every reasonable effort to obtain the report for the purpose of establishing whether your impairment meets 5.05F.
b. Acute encephalopathy. We will not evaluate your acute encephalopathy under 5.05F if it results from conditions other than chronic liver disease, such as vascular events and neoplastic diseases. We will evaluate these other causes of acute encephalopathy under the appropriate body system listings.
11. End stage liver disease (ESLD) documented by scores from the SSA Chronic Liver Disease (SSA CLD) calculation (5.05G).
a. We will use the SSA CLD score to evaluate your ESLD under 5.05G. We explain how we calculate the SSA CLD score in b. through g. of this section.
b. To calculate the SSA CLD score, we use a formula that includes three laboratory values: Serum total bilirubin (mg/dL), serum creatinine (mg/dL), and International Normalized Ratio (INR). The formula for the SSA CLD score calculation is:
9.57 × [Loge(serum creatinine mg/dL)] + 3.78 × [Loge(serum total bilirubin mg/dL)] + 11.2 × [Loge(INR)] + 6.43c. When we indicate “Loge” in the formula for the SSA CLD score calculation, we mean the “base e logarithm” or “natural logarithm” (ln) of a numerical laboratory value, not the “base 10 logarithm” or “common logarithm” (log) of the laboratory value, and not the actual laboratory value. For example, if an individual has laboratory values of serum creatinine 1.2 mg/dL, serum total bilirubin 2.2 mg/dL, and INR 1.0, we would compute the SSA CLD score as follows:
9.57 × [Loge(serum creatinine 1.2 mg/dL) = 0.182] + 3.78 × [Loge(serum total bilirubin 2.2 mg/dL) = 0.788] + 11.2 × [Loge(INR 1.0) = 0] + 6.43 ___ = 1.74 + 2.98 + 0 + 6.43 = 11.15, which is then rounded to an SSA CLD score of 11.d. For any SSA CLD score calculation, all of the required laboratory values must have been obtained within 30 days of each other. If there are multiple laboratory values within the 30-day interval for any given laboratory test (serum total bilirubin, serum creatinine, or INR), we will use the highest value for the SSA CLD score calculation. We will round all laboratory values less than 1.0 up to 1.0.
e. Listing 5.05G requires two SSA CLD scores. The laboratory values for the second SSA CLD score calculation must have been obtained at least 60 days after the latest laboratory value for the first SSA CLD score and within the required 6-month period. We will consider the date of each SSA CLD score to be the date of the first laboratory value used for its calculation.
f. If you are in renal failure or on dialysis within a week of any serum creatinine test in the period used for the SSA CLD calculation, we will use a serum creatinine of 4, which is the maximum serum creatinine level allowed in the calculation, to calculate your SSA CLD score.
g. If you have the two SSA CLD scores required by 5.05G, we will find that your impairment meets the criteria of the listing from at least the date of the first SSA CLD score.
12. Liver transplantation (5.09) may be performed for metabolic liver disease, progressive liver failure, life-threatening complications of liver disease, hepatic malignancy, and acute fulminant hepatitis (viral, drug-induced, or toxin-induced). We will consider you to be disabled for 1 year from the date of the transplantation. Thereafter, we will evaluate your residual impairment(s) by considering the adequacy of post-transplant liver function, the requirement for post-transplant antiviral therapy, the frequency and severity of rejection episodes, comorbid complications, and all adverse treatment effects.
E. How do we evaluate inflammatory bowel disease (IBD)?
1. Inflammatory bowel disease (5.06) includes, but is not limited to, Crohn's disease and ulcerative colitis. These disorders, while distinct entities, share many clinical, laboratory, and imaging findings, as well as similar treatment regimens. Remissions and exacerbations of variable duration are the hallmark of IBD. Crohn's disease may involve the entire alimentary tract from the mouth to the anus in a segmental, asymmetric fashion. Obstruction, stenosis, fistulization, perineal involvement, and extraintestinal manifestations are common. Crohn's disease is rarely curable and recurrence may be a lifelong problem, even after surgical resection. In contrast, ulcerative colitis only affects the colon. The inflammatory process may be limited to the rectum, extend proximally to include any contiguous segment, or involve the entire colon. Ulcerative colitis may be cured by total colectomy.
2. Symptoms and signs of IBD include diarrhea, fecal incontinence, rectal bleeding, abdominal pain, fatigue, fever, nausea, vomiting, arthralgia, abdominal tenderness, palpable abdominal mass (usually inflamed loops of bowel) and perineal disease. You may also have signs or laboratory findings indicating malnutrition, such as weight loss, edema, anemia, hypoalbuminemia, hypokalemia, hypocalcemia, or hypomagnesemia.
3. IBD may be associated with significant extraintestinal manifestations in a variety of body systems. These include, but are not limited to, involvement of the eye (for example, uveitis, episcleritis, iritis); hepatobiliary disease (for example, gallstones, primary sclerosing cholangitis); urologic disease (for example, kidney stones, obstructive hydronephrosis); skin involvement (for example, erythema nodosum, pyoderma gangrenosum); or non-destructive inflammatory arthritis. You may also have associated thromboembolic disorders or vascular disease. These manifestations may not correlate with the severity of your IBD. If your impairment does not meet any of the criteria of 5.06, we will consider the effects of your extraintestinal manifestations in determining whether you have an impairment(s) that meets or medically equals another listing, and we will also consider the effects of your extraintestinal manifestations when we assess your residual functional capacity.
4. Surgical diversion of the intestinal tract, including ileostomy and colostomy, does not preclude any gainful activity if you are able to maintain adequate nutrition and function of the stoma. However, if you are not able to maintain adequate nutrition, we will evaluate your impairment under 5.08.
F. How do we evaluate short bowel syndrome (SBS)?
1. Short bowel syndrome (5.07) is a disorder that occurs when ischemic vascular insults (for example, volvulus), trauma, or IBD complications require surgical resection of more than one-half of the small intestine, resulting in the loss of intestinal absorptive surface and a state of chronic malnutrition. The management of SBS requires long-term parenteral nutrition via an indwelling central venous catheter (central line); the process is often referred to as hyperalimentation or total parenteral nutrition (TPN). Individuals with SBS can also feed orally, with variable amounts of nutrients being absorbed through their remaining intestine. Over time, some of these individuals can develop additional intestinal absorptive surface, and may ultimately be able to be weaned off their parenteral nutrition.
2. Your impairment will continue to meet 5.07 as long as you remain dependent on daily parenteral nutrition via a central venous catheter for most of your nutritional requirements. Long-term complications of SBS and parenteral nutrition include central line infections (with or without septicemia), thrombosis, hepatotoxicity, gallstones, and loss of venous access sites. Intestinal transplantation is the only definitive treatment for individuals with SBS who remain chronically dependent on parenteral nutrition.
3. To document SBS, we need a copy of the operative report of intestinal resection, the summary of the hospitalization(s) including: Details of the surgical findings, medically appropriate postoperative imaging studies that reflect the amount of your residual small intestine, or if we cannot get one of these reports, other medical reports that include details of the surgical findings. We also need medical documentation that you are dependent on daily parenteral nutrition to provide most of your nutritional requirements.
G. How do we evaluate weight loss due to any digestive disorder?
1. In addition to the impairments specifically mentioned in these listings, other digestive disorders, such as esophageal stricture, pancreatic insufficiency, and malabsorption, may result in significant weight loss. We evaluate weight loss due to any digestive disorder under 5.08 by using the Body Mass Index (BMI). We also provide a criterion in 5.06B for lesser weight loss resulting from IBD.
2. BMI is the ratio of your weight to the square of your height. Calculation and interpretation of the BMI are independent of gender in adults.
a. We calculate BMI using inches and pounds, meters and kilograms, or centimeters and kilograms. We must have measurements of your weight and height without shoes for these calculations.
b. We calculate BMI using one of the following formulas:
H. What do we mean by the phrase “consider under a disability for 1 year”? We use the phrase “consider under a disability for 1 year” following a specific event in 5.02, 5.05A, and 5.09 to explain how long your impairment can meet the requirements of those particular listings. This phrase does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your impairment continues to meet a listing or is otherwise disabling. For example, if you have received a liver transplant, you may have become disabled before the transplant because of chronic liver disease. Therefore, we do not restrict our determination of the onset of disability to the date of the specified event. We will establish an onset date earlier than the date of the specified event if the evidence in your case record supports such a finding.
I. How do we evaluate impairments that do not meet one of the digestive disorder listings?
1. These listings are only examples of common digestive disorders that we consider severe enough to prevent you from doing any gainful activity. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system. For example, if you have hepatitis B or C and you are depressed, we will evaluate your impairment under 12.04.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926.) If your impairment(s) does not meet or medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. In this situation, we will proceed to the fourth, and if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920. When we decide whether you continue to be disabled, we use the rules in §§ 404.1594, 416.994, and 416.994a as appropriate.
5.01 Category of Impairments, Digestive System
5.02 Gastrointestinal hemorrhaging from any cause, requiring blood transfusion (with or without hospitalization) of at least 2 units of blood per transfusion, and occurring at least three times during a consecutive 6-month period. The transfusions must be at least 30 days apart within the 6-month period. Consider under a disability for 1 year following the last documented transfusion; thereafter, evaluate the residual impairment(s).
5.03-5.04 [Reserved]
5.05 Chronic liver disease, with:
A. Hemorrhaging from esophageal, gastric, or ectopic varices or from portal hypertensive gastropathy, demonstrated by endoscopy, x-ray, or other appropriate medically acceptable imaging, resulting in hemodynamic instability as defined in 5.00D5, and requiring hospitalization for transfusion of at least 2 units of blood. Consider under a disability for 1 year following the last documented transfusion; thereafter, evaluate the residual impairment(s).
ORB. Ascites or hydrothorax not attributable to other causes, despite continuing treatment as prescribed, present on at least two evaluations at least 60 days apart within a consecutive 6-month period. Each evaluation must be documented by:
1. Paracentesis or thoracentesis; or
2. Appropriate medically acceptable imaging or physical examination and one of the following:
a. Serum albumin of 3.0 g/dL or less; or
b. International Normalized Ratio (INR) of at least 1.5.
ORC. Spontaneous bacterial peritonitis with peritoneal fluid containing an absolute neutrophil count of at least 250 cells/mm 3.
ORD. Hepatorenal syndrome as described in 5.00D8, with one of the following:
1. Serum creatinine elevation of at least 2 mg/dL; or
2. Oliguria with 24-hour urine output less than 500 mL; or
3. Sodium retention with urine sodium less than 10 mEq per liter.
ORE. Hepatopulmonary syndrome as described in 5.00D9, with:
1. Arterial oxygenation (PaO2) on room air of:
a. 60 mm Hg or less, at test sites less than 3000 feet above sea level, or
b. 55 mm Hg or less, at test sites from 3000 to 6000 feet, or
c. 50 mm Hg or less, at test sites above 6000 feet; or
2. Documentation of intrapulmonary arteriovenous shunting by contrast-enhanced echocardiography or macroaggregated albumin lung perfusion scan.
ORF. Hepatic encephalopathy as described in 5.00D10, with 1 and either 2 or 3:
1. Documentation of abnormal behavior, cognitive dysfunction, changes in mental status, or altered state of consciousness (for example, confusion, delirium, stupor, or coma), present on at least two evaluations at least 60 days apart within a consecutive 6-month period; and
2. History of transjugular intrahepatic portosystemic shunt (TIPS) or any surgical portosystemic shunt: or
3. One of the following occurring on at least two evaluations at least 60 days apart within the same consecutive 6-month period as in F1:
a. Asterixis or other fluctuating physical neurological abnormalities; or
b. Electroencephalogram (EEG) demonstrating triphasic slow wave activity; or
c. Serum albumin of 3.0 g/dL or less; or
d. International Normalized Ratio (INR) of 1.5 or greater.
ORG. End stage liver disease with SSA CLD scores of 22 or greater calculated as described in 5.00D11. Consider under a disability from at least the date of the first score.
5.06 Inflammatory bowel disease (IBD) documented by endoscopy, biopsy, appropriate medically acceptable imaging, or operative findings with:
A. Obstruction of stenotic areas (not adhesions) in the small intestine or colon with proximal dilatation, confirmed by appropriate medically acceptable imaging or in surgery, requiring hospitalization for intestinal decompression or for surgery, and occurring on at least two occasions at least 60 days apart within a consecutive 6-month period;
ORB. Two of the following despite continuing treatment as prescribed and occurring within the same consecutive 6-month period:
1. Anemia with hemoglobin of less than 10.0 g/dL, present on at least two evaluations at least 60 days apart; or
2. Serum albumin of 3.0 g/dL or less, present on at least two evaluations at least 60 days apart; or
3. Clinically documented tender abdominal mass palpable on physical examination with abdominal pain or cramping that is not completely controlled by prescribed narcotic medication, present on at least two evaluations at least 60 days apart; or
4. Perineal disease with a draining abscess or fistula, with pain that is not completely controlled by prescribed narcotic medication, present on at least two evaluations at least 60 days apart; or
5. Involuntary weight loss of at least 10 percent from baseline, as computed in pounds, kilograms, or BMI, present on at least two evaluations at least 60 days apart; or
6. Need for supplemental daily enteral nutrition via a gastrostomy or daily parenteral nutrition via a central venous catheter.
5.07 Short bowel syndrome (SBS), due to surgical resection of more than one-half of the small intestine, with dependence on daily parenteral nutrition via a central venous catheter (see 5.00F).
5.08 Weight loss due to any digestive disorder despite continuing treatment as prescribed, with BMI of less than 17.50 calculated on at least two evaluations at least 60 days apart within a consecutive 6-month period.
5.09 Liver transplantation. Consider under a disability for 1 year following the date of transplantation; thereafter, evaluate the residual impairment(s) (see 5.00D12 and 5.00H).
6.00 Genitourinary disorders A. Which disorders do we evaluate under these listings?We evaluate genitourinary disorders resulting in chronic kidney disease (CKD). Examples of such disorders include chronic glomerulonephritis, hypertensive nephropathy, diabetic nephropathy, chronic obstructive uropathy, and hereditary nephropathies. We also evaluate nephrotic syndrome due to glomerular dysfunction under these listings.
B. What evidence do we need?1. We need evidence that documents the signs, symptoms, and laboratory findings of your CKD. This evidence should include reports of clinical examinations, treatment records, and documentation of your response to treatment. Laboratory findings, such as serum creatinine or serum albumin levels, may document your kidney function. We generally need evidence covering a period of at least 90 days unless we can make a fully favorable determination or decision without it.
2. Estimated glomerular filtration rate (eGFR). The eGFR is an estimate of the filtering capacity of the kidneys that takes into account serum creatinine concentration and other variables, such as your age, gender, and body size. If your medical evidence includes eGFR findings, we will consider them when we evaluate your CKD under 6.05.
3. Kidney or bone biopsy. If you have had a kidney or bone biopsy, we need a copy of the pathology report. When we cannot get a copy of the pathology report, we will accept a statement from an acceptable medical source verifying that a biopsy was performed and describing the results.
C. What other factors do we consider when we evaluate your genitourinary disorder?1. Chronic hemodialysis or peritoneal dialysis.
a. Dialysis is a treatment for CKD that uses artificial means to remove toxic metabolic byproducts from the blood. Hemodialysis uses an artificial kidney machine to clean waste products from the blood; peritoneal dialysis uses a dialyzing solution that is introduced into and removed from the abdomen (peritoneal cavity) either continuously or intermittently. Under 6.03, your ongoing dialysis must have lasted or be expected to last for a continuous period of at least 12 months. To satisfy the requirements in 6.03, we will accept a report from an acceptable medical source that describes your CKD and your current dialysis, and indicates that your dialysis will be ongoing.
b. If you are undergoing chronic hemodialysis or peritoneal dialysis, your CKD may meet our definition of disability before you started dialysis. We will determine the onset of your disability based on the facts in your case record.
2. Kidney transplant.
a. If you receive a kidney transplant, we will consider you to be disabled under 6.04 for 1 year from the date of transplant. After that, we will evaluate your residual impairment(s) by considering your post-transplant function, any rejection episodes you have had, complications in other body systems, and any adverse effects related to ongoing treatment.
b. If you received a kidney transplant, your CKD may meet our definition of disability before you received the transplant. We will determine the onset of your disability based on the facts in your case record.
3. Renal osteodystrophy. This condition is the bone degeneration resulting from chronic kidney disease-mineral and bone disorder (CKD-MBD). CKD-MBD occurs when the kidneys are unable to maintain the necessary levels of minerals, hormones, and vitamins required for bone structure and function. Under 6.05B1, “severe bone pain” means frequent or intractable (resistant to treatment) bone pain that interferes with physical activity or mental functioning.
4. Peripheral neuropathy. This disorder results when the kidneys do not adequately filter toxic substances from the blood. These toxins can adversely affect nerve tissue. The resulting neuropathy may affect peripheral motor or sensory nerves, or both, causing pain, numbness, tingling, and muscle weakness in various parts of the body. Under 6.05B2, the peripheral neuropathy must be a severe impairment. (See §§ 404.1520(c), 404.1521, 416.920(c), and 416.921 of this chapter.) It must also have lasted or be expected to last for a continuous period of at least 12 months.
5. Fluid overload syndrome. This condition occurs when excess sodium and water retention in the body due to CKD results in vascular congestion. Under 6.05B3, we need a description of a physical examination that documents signs and symptoms of vascular congestion, such as congestive heart failure, pleural effusion (excess fluid in the chest), ascites (excess fluid in the abdomen), hypertension, fatigue, shortness of breath, or peripheral edema.
6. Anasarca (generalized massive edema or swelling). Under 6.05B3 and 6.06B, we need a description of the extent of edema, including pretibial (in front of the tibia), periorbital (around the eyes), or presacral (in front of the sacrum) edema. We also need a description of any ascites, pleural effusion, or pericardial effusion.
7. Anorexia (diminished appetite) with weight loss. Anorexia is a frequent sign of CKD and can result in weight loss. We will use body mass index (BMI) to determine the severity of your weight loss under 6.05B4. (BMI is the ratio of your measured weight to the square of your measured height.) The formula for calculating BMI is in section 5.00G.
8. Complications of CKD. The hospitalizations in 6.09 may be for different complications of CKD. Examples of complications from CKD that may result in hospitalization include stroke, congestive heart failure, hypertensive crisis, or acute kidney failure requiring a short course of hemodialysis. If the CKD complication occurs during a hospitalization that was initially for a co-occurring condition, we will evaluate it under our rules for determining medical equivalence. (See §§ 404.1526 and 416.926 of this chapter.) We will evaluate co-occurring conditions, including those that result in hospitalizations, under the listings for the affected body system or under our rules for medical equivalence.
D. How do we evaluate disorders that do not meet one of the genitourinary listings?1. The listed disorders are only examples of common genitourinary disorders that we consider severe enough to prevent you from doing any gainful activity. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926 of this chapter.) Genitourinary disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not meet or medically equal the criteria of a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. We proceed to the fourth and, if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
6.01 Category of Impairments, Genitourinary Disorders6.03 Chronic kidney disease, with chronic hemodialysis or peritoneal dialysis (see 6.00C1).
6.04 Chronic kidney disease, with kidney transplant. Consider under a disability for 1 year following the transplant; thereafter, evaluate the residual impairment (see 6.00C2).
6.05 Chronic kidney disease, with impairment of kidney function, with A and B:
A. Reduced glomerular filtration evidenced by one of the following laboratory findings documented on at least two occasions at least 90 days apart during a consecutive 12-month period:
1. Serum creatinine of 4 mg/dL or greater; or
2. Creatinine clearance of 20 ml/min. or less; or
3. Estimated glomerular filtration rate (eGFR) of 20 ml/min/1.73m 2 or less.
ANDB. One of the following:
1. Renal osteodystrophy (see 6.00C3) with severe bone pain and imaging studies documenting bone abnormalities, such as osteitis fibrosa, osteomalacia, or pathologic fractures; or
2. Peripheral neuropathy (see 6.00C4); or
3. Fluid overload syndrome (see 6.00C5) documented by one of the following:
a. Diastolic hypertension greater than or equal to diastolic blood pressure of 110 mm Hg despite at least 90 consecutive days of prescribed therapy, documented by at least two measurements of diastolic blood pressure at least 90 days apart during a consecutive 12-month period; or
b. Signs of vascular congestion or anasarca (see 6.00C6) despite at least 90 consecutive days of prescribed therapy, documented on at least two occasions at least 90 days apart during a consecutive 12-month period; or
4. Anorexia with weight loss (see 6.00C7) determined by body mass index (BMI) of 18.0 or less, calculated on at least two occasions at least 90 days apart during a consecutive 12-month period.
6.06 Nephrotic syndrome, with A and B:
A. Laboratory findings as described in 1 or 2, documented on at least two occasions at least 90 days apart during a consecutive 12-month period:
1. Proteinuria of 10.0 g or greater per 24 hours; or
2. Serum albumin of 3.0 g/dL or less, and
a. Proteinuria of 3.5 g or greater per 24 hours; or
b. Urine total-protein-to-creatinine ratio of 3.5 or greater.
ANDB. Anasarca (see 6.00C6) persisting for at least 90 days despite prescribed treatment.
6.09 Complications of chronic kidney disease (see 6.00C8) requiring at least three hospitalizations within a consecutive 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
7.00 Hematological Disorders A. What hematological disorders do we evaluate under these listings?1. We evaluate non-malignant (non-cancerous) hematological disorders, such as hemolytic anemias (7.05), disorders of thrombosis and hemostasis (7.08), and disorders of bone marrow failure (7.10). These disorders disrupt the normal development and function of white blood cells, red blood cells, platelets, and clotting-factor proteins (factors).
2. We evaluate malignant (cancerous) hematological disorders, such as lymphoma, leukemia, and multiple myeloma, under the appropriate listings in 13.00, except for two lymphomas associated with human immunodeficiency virus (HIV) infection. We evaluate primary central nervous system lymphoma associated with HIV infection under 14.11B, and primary effusion lymphoma associated with HIV infection under 14.11C.
B. What evidence do we need to document that you have a hematological disorder?We need the following evidence to document that you have a hematological disorder:
1. A laboratory report of a definitive test that establishes a hematological disorder, signed by a physician; or
2. A laboratory report of a definitive test that establishes a hematological disorder that is not signed by a physician and a report from a physician that states you have the disorder; or
3. When we do not have a laboratory report of a definitive test, a persuasive report from a physician that a diagnosis of your hematological disorder was confirmed by appropriate laboratory analysis or other diagnostic method(s). To be persuasive, this report must state that you had the appropriate definitive laboratory test or tests for diagnosing your disorder and provide the results, or explain how your diagnosis was established by other diagnostic method(s) consistent with the prevailing state of medical knowledge and clinical practice.
4. We will make every reasonable effort to obtain the results of appropriate laboratory testing you have had. We will not purchase complex, costly, or invasive tests, such as tests of clotting-factor proteins, and bone marrow aspirations.
C. What are hemolytic anemias, and how do we evaluate them under 7.05?1. Hemolytic anemias, both congenital and acquired, are disorders that result in premature destruction of red blood cells (RBCs). Hemolytic disorders include abnormalities of hemoglobin structure (hemoglobinopathies), abnormal RBC enzyme content and function, and RBC membrane (envelope) defects that are congenital or acquired. The diagnosis of hemolytic anemia is based on hemoglobin electrophoresis or analysis of the contents of the RBC (enzymes) and membrane. Examples of congenital hemolytic anemias include sickle cell disease, thalassemia and their variants, and hereditary spherocytosis. Acquired hemolytic anemias may result from autoimmune disease (for example, systemic lupus erythematosus) or mechanical devices (for example, heart valves, intravascular patches).
2. The hospitalizations in 7.05B do not all have to be for the same complication of the hemolytic anemia. They may be for three different complications of the disorder. Examples of complications of hemolytic anemia that may result in hospitalization include osteomyelitis, painful (vaso-occlusive) crisis, pulmonary infections or infarctions, acute chest syndrome, pulmonary hypertension, chronic heart failure, gallbladder disease, hepatic (liver) failure, renal (kidney) failure, nephrotic syndrome, aplastic crisis, and stroke. We will count the hours you receive emergency treatment in a comprehensive sickle cell disease center immediately before the hospitalization if this treatment is comparable to the treatment provided in a hospital emergency department.
3. For 7.05C, we do not require hemoglobin to be measured during a period in which you are free of pain or other symptoms of your disorder. We will accept hemoglobin measurements made while you are experiencing complications of your hemolytic anemia.
4. 7.05D refers to the most serious type of beta thalassemia major in which the bone marrow cannot produce sufficient numbers of normal RBCs to maintain life. The only available treatments for beta thalassemia major are life-long RBC transfusions (sometimes called hypertransfusion) or bone marrow transplantation. For purposes of 7.05D, we do not consider prophylactic RBC transfusions to prevent strokes or other complications in sickle cell disease and its variants to be of equal significance to life-saving RBC transfusions for beta thalassemia major. However, we will consider the functional limitations associated with prophylactic RBC transfusions and any associated side effects (for example, iron overload) under 7.18 and any affected body system(s). We will also evaluate strokes and resulting complications under 11.00 and 12.00.
D. What are disorders of thrombosis and hemostasis, and how do we evaluate them under 7.08?1. Disorders of thrombosis and hemostasis include both clotting and bleeding disorders, and may be congenital or acquired. These disorders are characterized by abnormalities in blood clotting that result in hypercoagulation (excessive blood clotting) or hypocoagulation (inadequate blood clotting). The diagnosis of a thrombosis or hemostasis disorder is based on evaluation of plasma clotting-factor proteins (factors) and platelets. Protein C or protein S deficiency and Factor V Leiden are examples of hypercoagulation disorders. Hemophilia, von Willebrand disease, and thrombocytopenia are examples of hypocoagulation disorders. Acquired excessive blood clotting may result from blood protein defects and acquired inadequate blood clotting (for example, acquired hemophilia A) may be associated with inhibitor autoantibodies.
2. The hospitalizations in 7.08 do not all have to be for the same complication of a disorder of thrombosis and hemostasis. They may be for three different complications of the disorder. Examples of complications that may result in hospitalization include anemias, thromboses, embolisms, and uncontrolled bleeding requiring multiple factor concentrate infusions or platelet transfusions. We will also consider any surgery that you have, even if it is not related to your hematological disorder, to be a complication of your disorder of thrombosis and hemostasis if you require treatment with clotting-factor proteins (for example, factor VIII or factor IX) or anticoagulant medication to control bleeding or coagulation in connection with your surgery. We will count the hours you receive emergency treatment in a comprehensive hemophilia treatment center immediately before the hospitalization if this treatment is comparable to the treatment provided in a hospital emergency department.
E. What are disorders of bone marrow failure, and how do we evaluate them under 7.10?1. Disorders of bone marrow failure may be congenital or acquired, characterized by bone marrow that does not make enough healthy RBCs, platelets, or granulocytes (specialized types of white blood cells); there may also be a combined failure of these bone marrow-produced cells. The diagnosis is based on peripheral blood smears and bone marrow aspiration or bone marrow biopsy, but not peripheral blood smears alone. Examples of these disorders are myelodysplastic syndromes, aplastic anemia, granulocytopenia, and myelofibrosis. Acquired disorders of bone marrow failure may result from viral infections, chemical exposure, or immunologic disorders.
2. The hospitalizations in 7.10A do not all have to be for the same complication of bone marrow failure. They may be for three different complications of the disorder. Examples of complications that may result in hospitalization include uncontrolled bleeding, anemia, and systemic bacterial, viral, or fungal infections.
3. For 7.10B, the requirement of life-long RBC transfusions to maintain life in myelodysplastic syndromes or aplastic anemias has the same meaning as it does for beta thalassemia major. (See 7.00C4.)
F. How do we evaluate bone marrow or stem cell transplantation under 7.17?We will consider you to be disabled for 12 months from the date of bone marrow or stem cell transplantation, or we may consider you to be disabled for a longer period if you are experiencing any serious post-transplantation complications, such as graft-versus-host (GVH) disease, frequent infections after immunosuppressive therapy, or significant deterioration of organ systems. We do not restrict our determination of the onset of disability to the date of the transplantation in 7.17. We may establish an earlier onset date of disability due to your transplantation if evidence in your case record supports such a finding.
G. How do we use the functional criteria in 7.18?1. When we use the functional criteria in 7.18, we consider all relevant information in your case record to determine the impact of your hematological disorder on your ability to function independently, appropriately, effectively, and on a sustained basis in a work setting. Factors we will consider when we evaluate your functioning under 7.18 include, but are not limited to: Your symptoms, the frequency and duration of complications of your hematological disorder, periods of exacerbation and remission, and the functional impact of your treatment, including the side effects of your medication.
2. Repeated complications means that the complications occur on an average of three times a year, or once every 4 months, each lasting 2 weeks or more; or the complications do not last for 2 weeks but occur substantially more frequently than three times in a year or once every 4 months; or they occur less frequently than an average of three times a year or once every 4 months but last substantially longer than 2 weeks. Your impairment will satisfy this criterion regardless of whether you have the same kind of complication repeatedly, all different complications, or any other combination of complications; for example, two of the same kind of complication and a different one. You must have the required number of complications with the frequency and duration required in this section. Additionally, the complications must occur within the period we are considering in connection with your application or continuing disability review.
3. To satisfy the functional criteria in 7.18, your hematological disorder must result in a “marked” level of limitation in one of three general areas of functioning: Activities of daily living, social functioning, or difficulties in completing tasks due to deficiencies in concentration, persistence, or pace. Functional limitations may result from the impact of the disease process itself on your mental functioning, physical functioning, or both your mental and physical functioning. This limitation could result from persistent or intermittent symptoms, such as pain, severe fatigue, or malaise, resulting in a limitation of your ability to do a task, to concentrate, to persevere at a task, or to perform the task at an acceptable rate of speed. (Severe fatigue means a frequent sense of exhaustion that results in significant reduced physical activity or mental function. Malaise means frequent feelings of illness, bodily discomfort, or lack of well-being that result in significantly reduced physical activity or mental function.) You may also have limitations because of your treatment and its side effects.
4. Marked limitation means that the symptoms and signs of your hematological disorder interfere seriously with your ability to function. Although we do not require the use of such a scale, “marked” would be the fourth point on a five-point scale consisting of no limitation, mild limitation, moderate limitation, marked limitation, and extreme limitation. We do not define “marked” by a specific number of different activities of daily living or different behaviors in which your social functioning is impaired, or a specific number of tasks that you are able to complete, but by the nature and overall degree of interference with your functioning. You may have a marked limitation when several activities or functions are impaired, or even when only one is impaired. Additionally, you need not be totally precluded from performing an activity to have a marked limitation, as long as the degree of limitation interferes seriously with your ability to function independently, appropriately, and effectively. The term “marked” does not imply that you must be confined to bed, hospitalized, or in a nursing home.
5. Activities of daily living include, but are not limited to, such activities as doing household chores, grooming and hygiene, using a post office, taking public transportation, or paying bills. We will find that you have a “marked” limitation in activities of daily living if you have a serious limitation in your ability to maintain a household or take public transportation because of symptoms such as pain, severe fatigue, anxiety, or difficulty concentrating, caused by your hematological disorder (including complications of the disorder) or its treatment, even if you are able to perform some self-care activities.
6. Social functioning includes the capacity to interact with others independently, appropriately, effectively, and on a sustained basis. It includes the ability to communicate effectively with others. We will find that you have a “marked” limitation in maintaining social functioning if you have a serious limitation in social interaction on a sustained basis because of symptoms such as pain, severe fatigue, anxiety, or difficulty concentrating, or a pattern of exacerbation and remission, caused by your hematological disorder (including complications of the disorder) or its treatment, even if you are able to communicate with close friends or relatives.
7. Completing tasks in a timely manner involves the ability to sustain concentration, persistence, or pace to permit timely completion of tasks commonly found in work settings. We will find that you have a “marked” limitation in completing tasks if you have a serious limitation in your ability to sustain concentration or pace adequate to complete work-related tasks because of symptoms, such as pain, severe fatigue, anxiety, or difficulty concentrating caused by your hematological disorder (including complications of the disorder) or its treatment, even if you are able to do some routine activities of daily living.
H. How do we consider your symptoms, including your pain, severe fatigue, and malaise?Your symptoms, including pain, severe fatigue, and malaise, may be important factors in our determination whether your hematological disorder(s) meets or medically equals a listing, or in our determination whether you are otherwise able to work. We cannot consider your symptoms unless you have medical signs or laboratory findings showing the existence of a medically determinable impairment(s) that could reasonably be expected to produce the symptoms. If you have such an impairment(s), we will evaluate the intensity, persistence, and functional effects of your symptoms using the rules throughout 7.00 and in our other regulations. (See sections 404.1521, 404.1529, 416.921, and 416.929 of this chapter.) Additionally, when we assess the credibility of your complaints about your symptoms and their functional effects, we will not draw any inferences from the fact that you do not receive treatment or that you are not following treatment without considering all of the relevant evidence in your case record, including any explanations you provide that may explain why you are not receiving or following treatment.
I. How do we evaluate episodic events in hematological disorders?Some of the listings in this body system require a specific number of events within a consecutive 12-month period. (See 7.05, 7.08, and 7.10A.) When we use such criteria, a consecutive 12-month period means a period of 12 consecutive months, all or part of which must occur within the period we are considering in connection with your application or continuing disability review. These events must occur at least 30 days apart to ensure that we are evaluating separate events.
J. How do we evaluate hematological disorders that do not meet one of these listings?1. These listings are only common examples of hematological disorders that we consider severe enough to prevent a person from doing any gainful activity. If your disorder does not meet the criteria of any of these listings, we must consider whether you have a disorder that satisfies the criteria of a listing in another body system. For example, we will evaluate hemophilic joint deformity or bone or joint pain from myelofibrosis under 1.00; polycythemia vera under 3.00, 4.00, or 11.00; chronic iron overload resulting from repeated RBC transfusion (transfusion hemosiderosis) under 3.00, 4.00, or 5.00; and the effects of intracranial bleeding or stroke under 11.00 or 12.00.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See sections 404.1526 and 416.926 of this chapter.) Hematological disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. We proceed to the fourth, and, if necessary, the fifth steps of the sequential evaluation process in sections 404.1520 and 416.920. We use the rules in sections 404.1594, 416.994, and 416.994a of this chapter, as appropriate, when we decide whether you continue to be disabled.
7.01 Category of Impairments, Hematological Disorders
7.05 Hemolytic anemias, including sickle cell disease, thalassemia, and their variants (see 7.00C), with:
A. Documented painful (vaso-occlusive) crises requiring parenteral (intravenous or intramuscular) narcotic medication, occurring at least six times within a 12-month period with at least 30 days between crises.
OR
B. Complications of hemolytic anemia requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department or comprehensive sickle cell disease center immediately before the hospitalization (see 7.00C2).
OR
C. Hemoglobin measurements of 7.0 grams per deciliter (g/dL) or less, occurring at least three times within a 12-month period with at least 30 days between measurements.
OR
D. Beta thalassemia major requiring life-long RBC transfusions at least once every 6 weeks to maintain life (see 7.00C4).
7.08 Disorders of thrombosis and hemostasis, including hemophilia and thrombocytopenia (see 7.00D), with complications requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department or comprehensive hemophilia treatment center immediately before the hospitalization (see 7.00D2).
7.10 Disorders of bone marrow failure, including myelodysplastic syndromes, aplastic anemia, granulocytopenia, and myelofibrosis (see 7.00E), with:
A. Complications of bone marrow failure requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department immediately before the hospitalization (see 7.00E2).
OR
B. Myelodysplastic syndromes or aplastic anemias requiring life-long RBC transfusions at least once every 6 weeks to maintain life (see 7.00E3).
7.17 Hematological disorders treated by bone marrow or stem cell transplantation (see 7.00F). Consider under a disability for at least 12 consecutive months from the date of transplantation. After that, evaluate any residual impairment(s) under the criteria for the affected body system.
7.18 Repeated complications of hematological disorders (see 7.00G2), including those complications listed in 7.05, 7.08, and 7.10 but without the requisite findings for those listings, or other complications (for example, anemia, osteonecrosis, retinopathy, skin ulcers, silent central nervous system infarction, cognitive or other mental limitation, or limitation of joint movement), resulting in significant, documented symptoms or signs (for example, pain, severe fatigue, malaise, fever, night sweats, headaches, joint or muscle swelling, or shortness of breath), and one of the following at the marked level (see 7.00G4):
A. Limitation of activities of daily living (see 7.00G5).
B. Limitation in maintaining social functioning (see 7.00G6).
C. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace (see 7.00G7).
8.00 Skin DisordersA. What skin disorders do we evaluate with these listings? We use these listings to evaluate skin disorders that may result from hereditary, congenital, or acquired pathological processes. The kinds of impairments covered by these listings are: Ichthyosis, bullous diseases, chronic infections of the skin or mucous membranes, dermatitis, hidradenitis suppurativa, genetic photosensitivity disorders, and burns.
B. What documentation do we need? When we evaluate the existence and severity of your skin disorder, we generally need information about the onset, duration, frequency of flareups, and prognosis of your skin disorder; the location, size, and appearance of lesions; and, when applicable, history of exposure to toxins, allergens, or irritants, familial incidence, seasonal variation, stress factors, and your ability to function outside of a highly protective environment. To confirm the diagnosis, we may need laboratory findings (for example, results of a biopsy obtained independently of Social Security disability evaluation or blood tests) or evidence from other medically acceptable methods consistent with the prevailing state of medical knowledge and clinical practice.
C. How do we assess the severity of your skin disorder(s)? We generally base our assessment of severity on the extent of your skin lesions, the frequency of flareups of your skin lesions, how your symptoms (including pain) limit you, the extent of your treatment, and how your treatment affects you.
1. Extensive skin lesions. Extensive skin lesions are those that involve multiple body sites or critical body areas, and result in a very serious limitation. Examples of extensive skin lesions that result in a very serious limitation include but are not limited to:
a. Skin lesions that interfere with the motion of your joints and that very seriously limit your use of more than one extremity; that is, two upper extremities, two lower extremities, or one upper and one lower extremity.
b. Skin lesions on the palms of both hands that very seriously limit your ability to do fine and gross motor movements.
c. Skin lesions on the soles of both feet, the perineum, or both inguinal areas that very seriously limit your ability to ambulate.
2. Frequency of flareups. If you have skin lesions, but they do not meet the requirements of any of the listings in this body system, you may still have an impairment that prevents you from doing any gainful activity when we consider your condition over time, especially if your flareups result in extensive skin lesions, as defined in C1 of this section. Therefore, if you have frequent flareups, we may find that your impairment(s) is medically equal to one of these listings even though you have some periods during which your condition is in remission. We will consider how frequent and serious your flareups are, how quickly they resolve, and how you function between flareups to determine whether you have been unable to do any gainful activity for a continuous period of at least 12 months or can be expected to be unable to do any gainful activity for a continuous period of at least 12 months. We will also consider the frequency of your flareups when we determine whether you have a severe impairment and when we need to assess your residual functional capacity.
3. Symptoms (including pain). Symptoms (including pain) may be important factors contributing to the severity of your skin disorder(s). We assess the impact of symptoms as explained in §§ 404.1521, 404.1529, 416.921, and 416.929 of this chapter.
4. Treatment. We assess the effects of medication, therapy, surgery, and any other form of treatment you receive when we determine the severity and duration of your impairment(s). Skin disorders frequently respond to treatment; however, response to treatment can vary widely, with some impairments becoming resistant to treatment. Some treatments can have side effects that can in themselves result in limitations.
a. We assess the effects of continuing treatment as prescribed by determining if there is improvement in the symptoms, signs, and laboratory findings of your disorder, and if you experience side effects that result in functional limitations. To assess the effects of your treatment, we may need information about:
i. The treatment you have been prescribed (for example, the type, dosage, method, and frequency of administration of medication or therapy);
ii. Your response to the treatment;
iii. Any adverse effects of the treatment; and
iv. The expected duration of the treatment.
b. Because treatment itself or the effects of treatment may be temporary, in most cases sufficient time must elapse to allow us to evaluate the impact and expected duration of treatment and its side effects. Except under 8.07 and 8.08, you must follow continuing treatment as prescribed for at least 3 months before your impairment can be determined to meet the requirements of a skin disorder listing. (See 8.00H if you are not undergoing treatment or did not have treatment for 3 months.) We consider your specific response to treatment when we evaluate the overall severity of your impairment.
D. How do we assess impairments that may affect the skin and other body systems? When your impairment affects your skin and has effects in other body systems, we first evaluate the predominant feature of your impairment under the appropriate body system. Examples include, but are not limited to the following.
1. Tuberous sclerosis primarily affects the brain. The predominant features are seizures, which we evaluate under the neurological listings in 11.00, and developmental delays or other mental disorders, which we evaluate under the mental disorders listings in 12.00.
2. Malignant tumors of the skin (for example, malignant melanomas) are cancers, or neoplastic diseases, which we evaluate under the listings in 13.00.
3. Autoimmune disorders and other immune system disorders (for example, systemic lupus erythematosus (SLE), scleroderma, human immunodeficiency virus (HIV) infection, and Sjögren's syndrome) often involve more than one body system. We first evaluate these disorders under the immune system disorders listings in 14.00. We evaluate SLE under 14.02, scleroderma under 14.04, Sjögren's syndrome under 14.10, and HIV infection under 14.11.
4. Disfigurement or deformity resulting from skin lesions may result in loss of sight, hearing, speech, and the ability to chew (mastication). We evaluate these impairments and their effects under the special senses and speech listings in 2.00 and the digestive system listings in 5.00. Facial disfigurement or other physical deformities may also have effects we evaluate under the mental disorders listings in 12.00, such as when they affect mood or social functioning.
E. How do we evaluate genetic photosensitivity disorders?
1. Xeroderma pigmentosum (XP). When you have XP, your impairment meets the requirements of 8.07A if you have clinical and laboratory findings showing that you have the disorder. (See 8.00E3.) People who have XP have a lifelong hypersensitivity to all forms of ultraviolet light and generally lead extremely restricted lives in highly protective environments in order to prevent skin cancers from developing. Some people with XP also experience problems with their eyes, neurological problems, mental disorders, and problems in other body systems.
2. Other genetic photosensitivity disorders. Other genetic photosensitivity disorders may vary in their effects on different people, and may not result in an inability to engage in any gainful activity for a continuous period of at least 12 months. Therefore, if you have a genetic photosensitivity disorder other than XP (established by clinical and laboratory findings as described in 8.00E3), you must show that you have either extensive skin lesions or an inability to function outside of a highly protective environment to meet the requirements of 8.07B. You must also show that your impairment meets the duration requirement. By inability to function outside of a highly protective environment we mean that you must avoid exposure to ultraviolet light (including sunlight passing through windows and light from unshielded fluorescent bulbs), wear protective clothing and eyeglasses, and use opaque broad-spectrum sunscreens in order to avoid skin cancer or other serious effects. Some genetic photosensitivity disorders can have very serious effects in other body systems, especially special senses and speech (2.00), neurological (11.00), mental (12.00), and neoplastic (13.00). We will evaluate the predominant feature of your impairment under the appropriate body system, as explained in 8.00D.
3. Clinical and laboratory findings.
a. General. We need documentation from an acceptable medical source to establish that you have a medically determinable impairment. In general, we must have evidence of appropriate laboratory testing showing that you have XP or another genetic photosensitivity disorder. We will find that you have XP or another genetic photosensitivity disorder based on a report from an acceptable medical source indicating that you have the impairment, supported by definitive genetic laboratory studies documenting appropriate chromosomal changes, including abnormal DNA repair or another DNA or genetic abnormality specific to your type of photosensitivity disorder.
b. What we will accept as medical evidence instead of the actual laboratory report. When we do not have the actual laboratory report, we need evidence from an acceptable medical source that includes appropriate clinical findings for your impairment and that is persuasive that a positive diagnosis has been confirmed by appropriate laboratory testing at some time prior to our evaluation. To be persuasive, the report must state that the appropriate definitive genetic laboratory study was conducted and that the results confirmed the diagnosis. The report must be consistent with other evidence in your case record.
F. How do we evaluate burns? Electrical, chemical, or thermal burns frequently affect other body systems; for example, musculoskeletal, special senses and speech, respiratory, cardiovascular, renal, neurological, or mental. Consequently, we evaluate burns the way we evaluate other disorders that can affect the skin and other body systems, using the listing for the predominant feature of your impairment. For example, if your soft tissue injuries are under continuing surgical management (as defined in 1.00M), we will evaluate your impairment under 1.08. However, if your burns do not meet the requirements of 1.08 and you have extensive skin lesions that result in a very serious limitation (as defined in 8.00C1) that has lasted or can be expected to last for a continuous period of at least 12 months, we will evaluate them under 8.08.
G. How do we determine if your skin disorder(s) will continue at a disabling level of severity in order to meet the duration requirement? For all of these skin disorder listings except 8.07 and 8.08, we will find that your impairment meets the duration requirement if your skin disorder results in extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed. By persist, we mean that the longitudinal clinical record shows that, with few exceptions, your lesions have been at the level of severity specified in the listing. For 8.07A, we will presume that you meet the duration requirement. For 8.07B and 8.08, we will consider all of the relevant medical and other information in your case record to determine whether your skin disorder meets the duration requirement.
H. How do we assess your skin disorder(s) if your impairment does not meet the requirements of one of these listings?
1. These listings are only examples of common skin disorders that we consider severe enough to prevent you from engaging in any gainful activity. For most of these listings, if you do not have continuing treatment as prescribed, if your treatment has not lasted for at least 3 months, or if you do not have extensive skin lesions that have persisted for at least 3 months, your impairment cannot meet the requirements of these skin disorder listings. (This provision does not apply to 8.07 and 8.08.) However, we may still find that you are disabled because your impairment(s) meets the requirements of a listing in another body system or medically equals the severity of a listing. (See §§ 404.1526 and 416.926 of this chapter.) We may also find you disabled at the last step of the sequential evaluation process.
2. If you have not received ongoing treatment or do not have an ongoing relationship with the medical community despite the existence of a severe impairment(s), or if your skin lesions have not persisted for at least 3 months but you are undergoing continuing treatment as prescribed, you may still have an impairment(s) that meets a listing in another body system or that medically equals a listing. If you do not have an impairment(s) that meets or medically equals a listing, we will assess your residual functional capacity and proceed to the fourth and, if necessary, the fifth step of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. When we decide whether you continue to be disabled, we use the rules in §§ 404.1594 and 416.994 of this chapter.
8.01 Category of Impairments, Skin Disorders8.02 Ichthyosis, with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
8.03 Bullous disease (for example, pemphigus, erythema multiforme bullosum, epidermolysis bullosa, bullous pemphigoid, dermatitis herpetiformis), with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
8.04 Chronic infections of the skin or mucous membranes, with extensive fungating or extensive ulcerating skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
8.05 Dermatitis (for example, psoriasis, dyshidrosis, atopic dermatitis, exfoliative dermatitis, allergic contact dermatitis), with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
8.06 Hidradenitis suppurativa, with extensive skin lesions involving both axillae, both inguinal areas or the perineum that persist for at least 3 months despite continuing treatment as prescribed.
8.07 Genetic photosensitivity disorders, established as described in 8.00E.
A. Xeroderma pigmentosum. Consider the individual disabled from birth.
B. Other genetic photosensitivity disorders, with:
1. Extensive skin lesions that have lasted or can be expected to last for a continuous period of at least 12 months, or
2. Inability to function outside of a highly protective environment for a continuous period of at least 12 months (see 8.00E2).
8.08 Burns, with extensive skin lesions that have lasted or can be expected to last for a continuous period of at least 12 months (see 8.00F).
9.00 Endocrine DisordersA. What is an endocrine disorder?
An endocrine disorder is a medical condition that causes a hormonal imbalance. When an endocrine gland functions abnormally, producing either too much of a specific hormone (hyperfunction) or too little (hypofunction), the hormonal imbalance can cause various complications in the body. The major glands of the endocrine system are the pituitary, thyroid, parathyroid, adrenal, and pancreas.
B. How do we evaluate the effects of endocrine disorders? We evaluate impairments that result from endocrine disorders under the listings for other body systems. For example:
1. Pituitary gland disorders can disrupt hormone production and normal functioning in other endocrine glands and in many body systems. The effects of pituitary gland disorders vary depending on which hormones are involved. For example, when pituitary hypofunction affects water and electrolyte balance in the kidney and leads to diabetes insipidus, we evaluate the effects of recurrent dehydration under 6.00.
2. Thyroid gland disorders affect the sympathetic nervous system and normal metabolism. We evaluate thyroid-related changes in blood pressure and heart rate that cause arrhythmias or other cardiac dysfunction under 4.00; thyroid-related weight loss under 5.00; hypertensive cerebrovascular accidents (strokes) under 11.00; and cognitive limitations, mood disorders, and anxiety under 12.00.
3. Parathyroid gland disorders affect calcium levels in bone, blood, nerves, muscle, and other body tissues. We evaluate parathyroid-related osteoporosis and fractures under 1.00; abnormally elevated calcium levels in the blood (hypercalcemia) that lead to cataracts under 2.00; kidney failure under 6.00; and recurrent abnormally low blood calcium levels (hypocalcemia) that lead to increased excitability of nerves and muscles, such as tetany and muscle spasms, under 11.00.
4. Adrenal gland disorders affect bone calcium levels, blood pressure, metabolism, and mental status. We evaluate adrenal-related osteoporosis with fractures that compromises the ability to walk or to use the upper extremities under 1.00; adrenal-related hypertension that worsens heart failure or causes recurrent arrhythmias under 4.00; adrenal-related weight loss under 5.00; and mood disorders under 12.00.
5. Diabetes mellitus and other pancreatic gland disorders disrupt the production of several hormones, including insulin, that regulate metabolism and digestion. Insulin is essential to the absorption of glucose from the bloodstream into body cells for conversion into cellular energy. The most common pancreatic gland disorder is diabetes mellitus (DM). There are two major types of DM: type 1 and type 2. Both type 1 and type 2 DM are chronic disorders that can have serious disabling complications that meet the duration requirement. Type 1 DM - previously known as “juvenile diabetes” or “insulin-dependent diabetes mellitus” (IDDM) - is an absolute deficiency of insulin production that commonly begins in childhood and continues throughout adulthood. Treatment of type 1 DM always requires lifelong daily insulin. With type 2 DM - previously known as “adult-onset diabetes mellitus” or “non-insulin-dependent diabetes mellitus” (NIDDM) - the body's cells resist the effects of insulin, impairing glucose absorption and metabolism. Treatment of type 2 DM generally requires lifestyle changes, such as increased exercise and dietary modification, and sometimes insulin in addition to other medications. While both type 1 and type 2 DM are usually controlled, some persons do not achieve good control for a variety of reasons including, but not limited to, hypoglycemia unawareness, other disorders that can affect blood glucose levels, inability to manage DM due to a mental disorder, or inadequate treatment.
a. Hyperglycemia. Both types of DM cause hyperglycemia, which is an abnormally high level of blood glucose that may produce acute and long-term complications. Acute complications of hyperglycemia include diabetic ketoacidosis. Long-term complications of chronic hyperglycemia include many conditions affecting various body systems.
(i) Diabetic ketoacidosis (DKA). DKA is an acute, potentially life-threatening complication of DM in which the chemical balance of the body becomes dangerously hyperglycemic and acidic. It results from a severe insulin deficiency, which can occur due to missed or inadequate daily insulin therapy or in association with an acute illness. It usually requires hospital treatment to correct the acute complications of dehydration, electrolyte imbalance, and insulin deficiency. You may have serious complications resulting from your treatment, which we evaluate under the affected body system. For example, we evaluate cardiac arrhythmias under 4.00, intestinal necrosis under 5.00, and cerebral edema and seizures under 11.00. Recurrent episodes of DKA may result from mood or eating disorders, which we evaluate under 12.00.
(ii) Chronic hyperglycemia. Chronic hyperglycemia, which is longstanding abnormally high levels of blood glucose, leads to long-term diabetic complications by disrupting nerve and blood vessel functioning. This disruption can have many different effects in other body systems. For example, we evaluate diabetic peripheral neurovascular disease that leads to gangrene and subsequent amputation of an extremity under 1.00; diabetic retinopathy under 2.00; coronary artery disease and peripheral vascular disease under 4.00; diabetic gastroparesis that results in abnormal gastrointestinal motility under 5.00; diabetic nephropathy under 6.00; poorly healing bacterial and fungal skin infections under 8.00; diabetic peripheral and sensory neuropathies under 11.00; and cognitive impairments, depression, and anxiety under 12.00.
b. Hypoglycemia. Persons with DM may experience episodes of hypoglycemia, which is an abnormally low level of blood glucose. Most adults recognize the symptoms of hypoglycemia and reverse them by consuming substances containing glucose; however, some do not take this step because of hypoglycemia unawareness. Severe hypoglycemia can lead to complications, including seizures or loss of consciousness, which we evaluate under 11.00, or altered mental status and cognitive deficits, which we evaluate under 12.00.
C. How do we evaluate endocrine disorders that do not have effects that meet or medically equal the criteria of any listing in other body systems? If your impairment(s) does not meet or medically equal a listing in another body system, you may or may not have the residual functional capacity to engage in substantial gainful activity. In this situation, we proceed to the fourth and, if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920. When we decide whether you continue to be disabled, we use the rules in §§ 404.1594, 416.994, and 416.994a.
10.00 Congenital Disorders that Affect Multiple Body SystemsA. Which disorder do we evaluate under this body system? Although Down syndrome exists in non-mosaic and mosaic forms, we evaluate only non-mosaic Down syndrome under this body system.
B. What is non-mosaic Down syndrome? Non-mosaic Down syndrome is a genetic disorder. Most people with non-mosaic Down syndrome have three copies of chromosome 21 in all of their cells (chromosome 21 trisomy); some have an extra copy of chromosome 21 attached to a different chromosome in all of their cells (chromosome 21 translocation). Virtually all people with non-mosaic Down syndrome have characteristic facial or other physical features, delayed physical development, and intellectual disability. People with non-mosaic Down syndrome may also have congenital heart disease, impaired vision, hearing problems, and other disorders. We evaluate non-mosaic Down syndrome under 10.06. If you have non-mosaic Down syndrome documented as described in 10.00C, we consider you disabled from birth.
C. What evidence do we need to document non-mosaic Down syndrome under 10.06?1. Under 10.06A, we will find you disabled based on laboratory findings.
a. To find that your disorder meets 10.06A, we need a copy of the laboratory report of karyotype analysis, which is the definitive test to establish non-mosaic Down syndrome. We will not purchase karyotype analysis. We will not accept a fluorescence in situ hybridization (FISH) test because it does not distinguish between the mosaic and non-mosaic forms of Down syndrome.
b. If a physician (see §§ 404.1513(a)(1) and 416.913(a)(1) of this chapter) has not signed the laboratory report of karyotype analysis, the evidence must also include a physician's statement that you have Down syndrome.
c. For purposes of 10.06A, we do not require additional evidence stating that you have the distinctive facial or other physical features of Down syndrome.
2. If we do not have a laboratory report of karyotype analysis showing that you have non-mosaic Down syndrome, we may find you disabled under 10.06B or 10.06C.
a. Under 10.06B, we need a physician's report stating: (i) your karyotype diagnosis or evidence that documents your type of Down syndrome is consistent with prior karyotype analysis (for example, reference to a diagnosis of “trisomy 21”), and (ii) that you have the distinctive facial or other physical features of Down syndrome. We do not require a detailed description of the facial or other physical features of the disorder. However, we will not find that your disorder meets 10.06B if we have evidence - such as evidence of functioning inconsistent with the diagnosis - that indicates that you do not have non-mosaic Down syndrome.
b. If we do not have evidence of prior karyotype analysis (you did not have testing, or you had testing but we do not have information from a physician about the test results), we will find that your disorder meets 10.06C if we have: (i) a physician's report stating that you have the distinctive facial or other physical features of Down syndrome, and (ii) evidence that your functioning is consistent with a diagnosis of non-mosaic Down syndrome. This evidence may include medical or nonmedical information about your physical and mental abilities, including information about your education, work history, or the results of psychological testing. However, we will not find that your disorder meets 10.06C if we have evidence - such as evidence of functioning inconsistent with the diagnosis - that indicates that you do not have non-mosaic Down syndrome.
D. How do we evaluate mosaic Down syndrome and other congenital disorders that affect multiple body systems?1. Mosaic Down syndrome. Approximately 2 percent of people with Down syndrome have the mosaic form. In mosaic Down syndrome, there are some cells with an extra copy of chromosome 21 and other cells with the normal two copies of chromosome 21. Mosaic Down syndrome can be so slight as to be undetected clinically, but it can also be profound and disabling, affecting various body systems.
2. Other congenital disorders that affect multiple body systems. Other congenital disorders, such as congenital anomalies, chromosomal disorders, dysmorphic syndromes, inborn metabolic syndromes, and perinatal infectious diseases, can cause deviation from, or interruption of, the normal function of the body or can interfere with development. Examples of these disorders include both the juvenile and late-onset forms of Tay-Sachs disease, trisomy X syndrome (XXX syndrome), fragile X syndrome, phenylketonuria (PKU), caudal regression syndrome, and fetal alcohol syndrome. For these disorders and other disorders like them, the degree of deviation, interruption, or interference, as well as the resulting functional limitations and their progression, may vary widely from person to person and may affect different body systems.
3. Evaluating the effects of mosaic Down syndrome or another congenital disorder under the listings. When the effects of mosaic Down syndrome or another congenital disorder that affects multiple body systems are sufficiently severe we evaluate the disorder under the appropriate affected body system(s), such as musculoskeletal, special senses and speech, neurological, or mental disorders. Otherwise, we evaluate the specific functional limitations that result from the disorder under our other rules described in 10.00E.
E. What if your disorder does not meet a listing?If you have a severe medically determinable impairment(s) that does not meet a listing, we will consider whether your impairment(s) medically equals a listing. See §§ 404.1526 and 416.926 of this chapter. If your impairment(s) does not meet or medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. We proceed to the fourth, and if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
10.01 Category of Impairments, Congenital Disorders That Affect Multiple Body Systems10.06 Non-mosaic Down syndrome (chromosome 21 trisomy or chromosome 21 translocation), documented by:
A. A laboratory report of karyotype analysis signed by a physician, or both a laboratory report of karyotype analysis not signed by a physician and a statement by a physician that you have Down syndrome (see 10.00C1), or
B. A physician's report stating that you have chromosome 21 trisomy or chromosome 21 translocation consistent with prior karyotype analysis with the distinctive facial or other physical features of Down syndrome (see 10.00C2a), or
C. A physician's report stating that you have Down syndrome with the distinctive facial or other physical features and evidence demonstrating that you function at a level consistent with non-mosaic Down syndrome (see 10.00C2b).
11.00 Neurological DisordersA. Which neurological disorders do we evaluate under these listings? We evaluate epilepsy, amyotrophic lateral sclerosis, coma or persistent vegetative state (PVS), and neurological disorders that cause disorganization of motor function, bulbar and neuromuscular dysfunction, communication impairment, or a combination of limitations in physical and mental functioning. We evaluate neurological disorders that may manifest in a combination of limitations in physical and mental functioning. For example, if you have a neurological disorder that causes mental limitations, such as Huntington's disease or early-onset Alzheimer's disease, which may limit executive functioning (e.g., regulating attention, planning, inhibiting responses, decision-making), we evaluate your limitations using the functional criteria under these listings (see 11.00G). Under this body system, we evaluate the limitations resulting from the impact of the neurological disease process itself. If your neurological disorder results in only mental impairment or if you have a co-occurring mental condition that is not caused by your neurological disorder (for example, dementia), we will evaluate your mental impairment under the mental disorders body system, 12.00.
B. What evidence do we need to document your neurological disorder?
1. We need both medical and non-medical evidence (signs, symptoms, and laboratory findings) to assess the effects of your neurological disorder. Medical evidence should include your medical history, examination findings, relevant laboratory tests, and the results of imaging. Imaging refers to medical imaging techniques, such as x-ray, computerized tomography (CT), magnetic resonance imaging (MRI), and electroencephalography (EEG). The imaging must be consistent with the prevailing state of medical knowledge and clinical practice as the proper technique to support the evaluation of the disorder. In addition, the medical evidence may include descriptions of any prescribed treatment and your response to it. We consider non-medical evidence such as statements you or others make about your impairments, your restrictions, your daily activities, or your efforts to work.
2. We will make every reasonable effort to obtain the results of your laboratory and imaging evidence. When the results of any of these tests are part of the existing evidence in your case record, we will evaluate the test results and all other relevant evidence. We will not purchase imaging, or other diagnostic tests, or laboratory tests that are complex, may involve significant risk, or that are invasive. We will not routinely purchase tests that are expensive or not readily available.
C. How do we consider adherence to prescribed treatment in neurological disorders? In 11.02 (Epilepsy), 11.06 (Parkinsonian syndrome), and 11.12 (Myasthenia gravis), we require that limitations from these neurological disorders exist despite adherence to prescribed treatment. “Despite adherence to prescribed treatment” means that you have taken medication(s) or followed other treatment procedures for your neurological disorder(s) as prescribed by a physician for three consecutive months but your impairment continues to meet the other listing requirements despite this treatment. You may receive your treatment at a health care facility that you visit regularly, even if you do not see the same physician on each visit.
D. What do we mean by disorganization of motor function?
1. Disorganization of motor function means interference, due to your neurological disorder, with movement of two extremities; i.e., the lower extremities, or upper extremities (including fingers, wrists, hands, arms, and shoulders). By two extremities we mean both lower extremities, or both upper extremities, or one upper extremity and one lower extremity. All listings in this body system, except for 11.02 (Epilepsy), 11.10 (Amyotrophic lateral sclerosis), and 11.20 (Coma and persistent vegetative state), include criteria for disorganization of motor function that results in an extreme limitation in your ability to:
a. Stand up from a seated position; or
b. Balance while standing or walking; or
c. Use the upper extremities (including fingers, wrists, hands, arms, and shoulders).
2. Extreme limitation means the inability to stand up from a seated position, maintain balance in a standing position and while walking, or use your upper extremities to independently initiate, sustain, and complete work-related activities. The assessment of motor function depends on the degree of interference with standing up; balancing while standing or walking; or using the upper extremities (including fingers, hands, arms, and shoulders).
a. Inability to stand up from a seated position means that once seated you are unable to stand and maintain an upright position without the assistance of another person or the use of an assistive device, such as a walker, two crutches, or two canes.
b. Inability to maintain balance in a standing position means that you are unable to maintain an upright position while standing or walking without the assistance of another person or an assistive device, such as a walker, two crutches, or two canes.
c. Inability to use your upper extremities means that you have a loss of function of both upper extremities (including fingers, wrists, hands, arms, and shoulders) that very seriously limits your ability to independently initiate, sustain, and complete work-related activities involving fine and gross motor movements. Inability to perform fine and gross motor movements could include not being able to pinch, manipulate, and use your fingers; or not being able to use your hands, arms, and shoulders to perform gross motor movements, such as handling, gripping, grasping, holding, turning, and reaching; or not being able to engage in exertional movements such a lifting, carrying, pushing, and pulling.
E. How do we evaluate communication impairments under these listings? We must have a description of a recent comprehensive evaluation including all areas of communication, performed by an acceptable medical source, to document a communication impairment associated with a neurological disorder. A communication impairment may occur when a medically determinable neurological impairment results in dysfunction in the parts of the brain responsible for speech and language. We evaluate communication impairments associated with neurological disorders under 11.04A, 11.07C, or 11.11B. We evaluate communication impairments due to non-neurological disorders under 2.09.
1. Under 11.04A, we need evidence documenting that your central nervous system vascular accident or insult (CVA) and sensory or motor aphasia have resulted in ineffective speech or communication. Ineffective speech or communication means there is an extreme limitation in your ability to understand or convey your message in simple spoken language resulting in your inability to demonstrate basic communication skills, such as following one-step commands or telling someone about your basic personal needs without assistance.
2. Under 11.07C, we need evidence documenting that your cerebral palsy has resulted in significant interference in your ability to speak, hear, or see. We will find you have “significant interference” in your ability to speak, hear, or see if your signs, such as aphasia, strabismus, or sensorineural hearing loss, seriously limit your ability to communicate on a sustained basis.
3. Under 11.11B, we need evidence documenting that your post-polio syndrome has resulted in the inability to produce intelligible speech.
F. What do we mean by bulbar and neuromuscular dysfunction? The bulbar region of the brain is responsible for controlling the bulbar muscles in the throat, tongue, jaw, and face. Bulbar and neuromuscular dysfunction refers to weakness in these muscles, resulting in breathing, swallowing, and speaking impairments. Listings 11.11 (Post-polio syndrome), 11.12 (Myasthenia gravis), and 11.22 (Motor neuron disorders other than ALS) include criteria for evaluating bulbar and neuromuscular dysfunction. If your neurological disorder has resulted in a breathing disorder, we may evaluate that condition under the respiratory system, 3.00.
G. How do we evaluate limitations in physical and mental functioning under these listings?
1. Neurological disorders may manifest in a combination of limitations in physical and mental functioning. We consider all relevant information in your case record to determine the effects of your neurological disorder on your physical and mental functioning. To satisfy the requirement described under 11.00G, your neurological disorder must result in a marked limitation in physical functioning and a marked limitation in at least one of four areas of mental functioning: Understanding, remembering, or applying information; interacting with others; concentrating, persisting, or maintaining pace; or adapting or managing oneself. If your neurological disorder results in an extreme limitation in at least one of the four areas of mental functioning, or results in marked limitation in at least two of the four areas of mental functioning, but you do not have at least a marked limitation in your physical functioning, we will consider whether your condition meets or medically equals one of the mental disorders body system listings, 12.00.
2. Marked Limitation. To satisfy the requirements of the functional criteria, your neurological disorder must result in a marked limitation in physical functioning and a marked limitation in one of the four areas of mental functioning (see 11.00G3). Although we do not require the use of such a scale, “marked” would be the fourth point on a five-point scale consisting of no limitation, mild limitation, moderate limitation, marked limitation, and extreme limitation. We consider the nature and overall degree of interference with your functioning. The term “marked” does not require that you must be confined to bed, hospitalized, or in a nursing home.
a. Marked limitation and physical functioning. For this criterion, a marked limitation means that, due to the signs and symptoms of your neurological disorder, you are seriously limited in the ability to independently initiate, sustain, and complete work-related physical activities (see 11.00G3). You may have a marked limitation in your physical functioning when your neurological disease process causes persistent or intermittent symptoms that affect your abilities to independently initiate, sustain, and complete work-related activities, such as standing, balancing, walking, using both upper extremities for fine and gross movements, or results in limitations in using one upper and one lower extremity. The persistent and intermittent symptoms must result in a serious limitation in your ability to do a task or activity on a sustained basis. We do not define “marked” by a specific number of different physical activities or tasks that demonstrate your ability, but by the overall effects of your neurological symptoms on your ability to perform such physical activities on a consistent and sustained basis. You need not be totally precluded from performing a function or activity to have a marked limitation, as long as the degree of limitation seriously limits your ability to independently initiate, sustain, and complete work-related physical activities.
b. Marked limitation and mental functioning. For this criterion, a marked limitation means that, due to the signs and symptoms of your neurological disorder, you are seriously limited in the ability to function independently, appropriately, effectively, and on a sustained basis in work settings (see 11.03G3). We do not define “marked” by a specific number of mental activities, such as: The number of activities that demonstrate your ability to understand, remember, and apply information; the number of tasks that demonstrate your ability to interact with others; a specific number of tasks that demonstrate you are able to concentrate, persist or maintain pace; or a specific number of tasks that demonstrate you are able to manage yourself. You may have a marked limitation in your mental functioning when several activities or functions are impaired, or even when only one is impaired. You need not be totally precluded from performing an activity to have a marked limitation, as long as the degree of limitation seriously limits your ability to function independently, appropriately, and effectively on a sustained basis, and complete work-related mental activities.
3. Areas of physical and mental functioning.
a. Physical functioning. Examples of this criterion include specific motor abilities, such as independently initiating, sustaining, and completing the following activities: Standing up from a seated position, balancing while standing or walking, or using both your upper extremities for fine and gross movements (see 11.00D). Physical functioning may also include functions of the body that support motor abilities, such as the abilities to see, breathe, and swallow (see 11.00E and 11.00F). Examples of when your limitation in seeing, breathing, or swallowing may, on its own, rise to a “marked” limitation include: Prolonged and uncorrectable double vision causing difficulty with balance; prolonged difficulty breathing requiring the use of a prescribed assistive breathing device, such as a portable continuous positive airway pressure machine; or repeated instances, occurring at least weekly, of aspiration without causing aspiration pneumonia. Alternatively, you may have a combination of limitations due to your neurological disorder that together rise to a “marked” limitation in physical functioning. We may also find that you have a “marked” limitation in this area if, for example, your symptoms, such as pain or fatigue (see 11.00T), as documented in your medical record, and caused by your neurological disorder or its treatment, seriously limit your ability to independently initiate, sustain, and complete these work-related motor functions, or the other physical functions or physiological processes that support those motor functions. We may also find you seriously limited in an area if, while you retain some ability to perform the function, you are unable to do so consistently and on a sustained basis. The limitation in your physical functioning must last or be expected to last at least 12 months. These examples illustrate the nature of physical functioning. We do not require documentation of all of the examples.
b. Mental functioning.
(i) Understanding, remembering, or applying information. This area of mental functioning refers to the abilities to learn, recall, and use information to perform work activities. Examples include: Understanding and learning terms, instructions, procedures; following one- or two-step oral instructions to carry out a task; describing work activity to someone else; asking and answering questions and providing explanations; recognizing a mistake and correcting it; identifying and solving problems; sequencing multi-step activities; and using reason and judgment to make work-related decisions. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
(ii) Interacting with others. This area of mental functioning refers to the abilities to relate to and work with supervisors, co-workers, and the public. Examples include: Cooperating with others; asking for help when needed; handling conflicts with others; stating your own point of view; initiating or sustaining conversation; understanding and responding to social cues (physical, verbal, emotional); responding to requests, suggestions, criticism, correction, and challenges; and keeping social interactions free of excessive irritability, sensitivity, argumentativeness, or suspiciousness. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
(iii) Concentrating, persisting, or maintaining pace. This area of mental functioning refers to the abilities to focus attention on work activities and to stay on-task at a sustained rate. Examples include: Initiating and performing a task that you understand and know how to do; working at an appropriate and consistent pace; completing tasks in a timely manner; ignoring or avoiding distractions while working; changing activities or work settings without being disruptive; working close to or with others without interrupting or distracting them; sustaining an ordinary routine and regular attendance at work; and working a full day without needing more than the allotted number or length of rest periods during the day. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
(iv) Adapting or managing oneself. This area of mental functioning refers to the abilities to regulate emotions, control behavior, and maintain well-being in a work setting. Examples include: Responding to demands; adapting to changes; managing your psychologically based symptoms; distinguishing between acceptable and unacceptable work performance; setting realistic goals; making plans for yourself independently of others; maintaining personal hygiene and attire appropriate to a work setting; and being aware of normal hazards and taking appropriate precautions. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
4. Signs and symptoms of your disorder and the effects of treatment.
a. We will consider your signs and symptoms and how they affect your ability to function in the work place. When we evaluate your functioning, we will consider whether your signs and symptoms are persistent or intermittent, how frequently they occur and how long they last, their intensity, and whether you have periods of exacerbation and remission.
b. We will consider the effectiveness of treatment in improving the signs, symptoms, and laboratory findings related to your neurological disorder, as well as any aspects of treatment that may interfere with your ability to function. We will consider, for example: The effects of medications you take (including side effects); the time-limited efficacy of some medications; the intrusiveness, complexity, and duration of your treatment (for example, the dosing schedule or need for injections); the effects of treatment, including medications, therapy, and surgery, on your functioning; the variability of your response to treatment; and any drug interactions.
H. What is epilepsy, and how do we evaluate it under 11.02?
1. Epilepsy is a pattern of recurrent and unprovoked seizures that are manifestations of abnormal electrical activity in the brain. There are various types of generalized and “focal” or partial seizures. However, psychogenic nonepileptic seizures and pseudoseizures are not epileptic seizures for the purpose of 11.02. We evaluate psychogenic seizures and pseudoseizures under the mental disorders body system, 12.00. In adults, the most common potentially disabling seizure types are generalized tonic-clonic seizures and dyscognitive seizures (formerly complex partial seizures).
a. Generalized tonic-clonic seizures are characterized by loss of consciousness accompanied by a tonic phase (sudden muscle tensing causing the person to lose postural control) followed by a clonic phase (rapid cycles of muscle contraction and relaxation, also called convulsions). Tongue biting and incontinence may occur during generalized tonic-clonic seizures, and injuries may result from falling.
b. Dyscognitive seizures are characterized by alteration of consciousness without convulsions or loss of muscle control. During the seizure, blank staring, change of facial expression, and automatisms (such as lip smacking, chewing or swallowing, or repetitive simple actions, such as gestures or verbal utterances) may occur. During its course, a dyscognitive seizure may progress into a generalized tonic-clonic seizure (see 11.00H1a).
2. Description of seizure. We require at least one detailed description of your seizures from someone, preferably a medical professional, who has observed at least one of your typical seizures. If you experience more than one type of seizure, we require a description of each type.
3. Serum drug levels. We do not require serum drug levels; therefore, we will not purchase them. However, if serum drug levels are available in your medical records, we will evaluate them in the context of the other evidence in your case record.
4. Counting seizures. The period specified in 11.02A, B, C, or D cannot begin earlier than one month after you began prescribed treatment. The required number of seizures must occur within the period we are considering in connection with your application or continuing disability review. When we evaluate the frequency of your seizures, we also consider your adherence to prescribed treatment (see 11.00C). When we determine the number of seizures you have had in the specified period, we will:
a. Count multiple seizures occurring in a 24-hour period as one seizure.
b. Count status epilepticus (a continuous series of seizures without return to consciousness between seizures) as one seizure.
c. Count a dyscognitive seizure that progresses into a generalized tonic-clonic seizure as one generalized tonic-clonic seizure.
d. We do not count seizures that occur during a period when you are not adhering to prescribed treatment without good reason. When we determine that you had good reason for not adhering to prescribed treatment, we will consider your physical, mental, educational, and communicative limitations (including any language barriers). We will consider you to have good reason for not following prescribed treatment if, for example, the treatment is very risky for you due to its consequences or unusual nature, or if you are unable to afford prescribed treatment that you are willing to accept, but for which no free community resources are available. We will follow guidelines found in our policy, such as §§ 404.1530(c) and 416.930(c) of this chapter, when we determine whether you have a good reason for not adhering to prescribed treatment.
e. We do not count psychogenic nonepileptic seizures or pseudoseizures under 11.02. We evaluate these seizures under the mental disorders body system, 12.00.
5. Electroencephalography (EEG) testing. We do not require EEG test results; therefore, we will not purchase them. However, if EEG test results are available in your medical records, we will evaluate them in the context of the other evidence in your case record.
I. What is vascular insult to the brain, and how do we evaluate it under 11.04?
1. Vascular insult to the brain (cerebrum, cerebellum, or brainstem), commonly referred to as stroke or cerebrovascular accident (CVA), is brain cell death caused by an interruption of blood flow within or leading to the brain, or by a hemorrhage from a ruptured blood vessel or aneurysm in the brain. If you have a vision impairment resulting from your vascular insult, we may evaluate that impairment under the special senses body system, 2.00.
2. We need evidence of sensory or motor aphasia that results in ineffective speech or communication under 11.04A (see 11.00E). We may evaluate your communication impairment under listing 11.04C if you have marked limitation in physical functioning and marked limitation in one of the four areas of mental functioning.
3. We generally need evidence from at least 3 months after the vascular insult to evaluate whether you have disorganization of motor functioning under 11.04B, or the impact that your disorder has on your physical and mental functioning under 11.04C. In some cases, evidence of your vascular insult is sufficient to allow your claim within 3 months post-vascular insult. If we are unable to allow your claim within 3 months after your vascular insult, we will defer adjudication of the claim until we obtain evidence of your neurological disorder at least 3 months post-vascular insult.
J. What are benign brain tumors, and how do we evaluate them under 11.05? Benign brain tumors are noncancerous (nonmalignant) abnormal growths of tissue in or on the brain that invade healthy brain tissue or apply pressure on the brain or cranial nerves. We evaluate their effects on your functioning as discussed in 11.00D and 11.00G. We evaluate malignant brain tumors under the cancer body system in 13.00. If you have a vision impairment resulting from your benign brain tumor, we may evaluate that impairment under the special senses body system, 2.00.
K. What is Parkinsonian syndrome, and how do we evaluate it under 11.06? Parkinsonian syndrome is a term that describes a group of chronic, progressive movement disorders resulting from loss or decline in the function of dopamine-producing brain cells. Dopamine is a neurotransmitter that regulates muscle movement throughout the body. When we evaluate your Parkinsonian syndrome, we will consider your adherence to prescribed treatment (see 11.00C).
L. What is cerebral palsy, and how do we evaluate it under 11.07?
1. Cerebral palsy (CP) is a term that describes a group of static, nonprogressive disorders caused by abnormalities within the brain that disrupt the brain's ability to control movement, muscle coordination, and posture. The resulting motor deficits manifest very early in a person's development, with delayed or abnormal progress in attaining developmental milestones. Deficits may become more obvious as the person grows and matures over time.
2. We evaluate your signs and symptoms, such as ataxia, spasticity, flaccidity, athetosis, chorea, and difficulty with precise movements when we determine your ability to stand up, balance, walk, or perform fine and gross motor movements. We will also evaluate your signs, such as dysarthria and apraxia of speech, and receptive and expressive language problems when we determine your ability to communicate.
3. We will consider your other impairments or signs and symptoms that develop secondary to the disorder, such as post-impairment syndrome (a combination of pain, fatigue, and weakness due to muscle abnormalities); overuse syndromes (repetitive motion injuries); arthritis; abnormalities of proprioception (perception of the movements and position of the body); abnormalities of stereognosis (perception and identification of objects by touch); learning problems; anxiety; and depression.
M. What are spinal cord disorders, and how do we evaluate them under 11.08?
1. Spinal cord disorders may be congenital or caused by injury to the spinal cord. Motor signs and symptoms of spinal cord disorders include paralysis, flaccidity, spasticity, and weakness.
2. Spinal cord disorders with complete loss of function (11.08A) addresses spinal cord disorders that result in a complete lack of motor, sensory, and autonomic function of the affected part(s) of the body.
3. Spinal cord disorders with disorganization of motor function (11.08B) addresses spinal cord disorders that result in less than a complete loss of function of the affected part(s) of the body, reducing, but not eliminating, motor, sensory, and autonomic function.
4. When we evaluate your spinal cord disorder, we generally need evidence from at least 3 months after your symptoms began in order to evaluate your disorganization of motor function. In some cases, evidence of your spinal cord disorder may be sufficient to allow your claim within 3 months after the spinal cord disorder. If the medical evidence demonstrates total cord transection causing a loss of motor and sensory functions below the level of injury, we will not wait 3 months but will make the allowance decision immediately.
N. What is multiple sclerosis, and how do we evaluate it under 11.09?
1. Multiple sclerosis (MS) is a chronic, inflammatory, degenerative disorder that damages the myelin sheath surrounding the nerve fibers in the brain and spinal cord. The damage disrupts the normal transmission of nerve impulses within the brain and between the brain and other parts of the body, causing impairment in muscle coordination, strength, balance, sensation, and vision. There are several forms of MS, ranging from mildly to highly aggressive. Milder forms generally involve acute attacks (exacerbations) with partial or complete recovery from signs and symptoms (remissions). Aggressive forms generally exhibit a steady progression of signs and symptoms with few or no remissions. The effects of all forms vary from person to person.
2. We evaluate your signs and symptoms, such as flaccidity, spasticity, spasms, incoordination, imbalance, tremor, physical fatigue, muscle weakness, dizziness, tingling, and numbness when we determine your ability to stand up, balance, walk, or perform fine and gross motor movements. When determining whether you have limitations of physical and mental functioning, we will consider your other impairments or signs and symptoms that develop secondary to the disorder, such as fatigue; visual loss; trouble sleeping; impaired attention, concentration, memory, or judgment; mood swings; and depression. If you have a vision impairment resulting from your MS, we may evaluate that impairment under the special senses body system, 2.00.
O. What is amyotrophic lateral sclerosis, and how do we evaluate it under 11.10? Amyotrophic lateral sclerosis (ALS) is a type of motor neuron disorder that rapidly and progressively attacks the nerve cells responsible for controlling voluntary muscles. We establish ALS under 11.10 when you have a documented diagnosis of ALS. We require documentation based on generally accepted methods consistent with the prevailing state of medical knowledge and clinical practice. We require laboratory testing to establish the diagnosis when the clinical findings of upper and lower motor neuron disease are not present in three or more regions. Electrophysiological studies, such as nerve conduction velocity studies and electromyography (EMG), may support your diagnosis of ALS; however, we will not purchase these studies.
P. What are neurodegenerative disorders of the central nervous system, such as Huntington's disease, Friedreich's ataxia, and spinocerebellar degeneration, and how do we evaluate them under 11.17? Neurodegenerative disorders of the central nervous system are disorders characterized by progressive and irreversible degeneration of neurons or their supporting cells. Over time, these disorders impair many of the body's motor, cognitive, and other mental functions. We consider neurodegenerative disorders of the central nervous system under 11.17 that we do not evaluate elsewhere in section 11.00, such as Huntington's disease (HD), Friedreich's ataxia, spinocerebellar degeneration, Creutzfeldt-Jakob disease (CJD), progressive supranuclear palsy (PSP), early-onset Alzheimer's disease, and frontotemporal dementia (Pick's disease). When these disorders result in solely cognitive and other mental function effects, we will evaluate the disorder under the mental disorder listings.
Q. What is traumatic brain injury, and how do we evaluate it under 11.18?
1. Traumatic brain injury (TBI) is damage to the brain resulting from skull fracture, collision with an external force leading to a closed head injury, or penetration by an object that enters the skull and makes contact with brain tissue. We evaluate TBI that results in coma or persistent vegetative state (PVS) under 11.20.
2. We generally need evidence from at least 3 months after the TBI to evaluate whether you have disorganization of motor function under 11.18A or the impact that your disorder has on your physical and mental functioning under 11.18B. In some cases, evidence of your TBI is sufficient to determine disability within 3 months post-TBI. If we are unable to allow your claim within 3 months post-TBI, we will defer adjudication of the claim until we obtain evidence of your neurological disorder at least 3 months post-TBI. If a finding of disability still is not possible at that time, we will again defer adjudication of the claim until we obtain evidence at least 6 months after your TBI.
R. What are coma and persistent vegetative state, and how do we evaluate them under 11.20? Coma is a state of unconsciousness in which a person does not exhibit a sleep/wake cycle, and is unable to perceive or respond to external stimuli. People who do not fully emerge from coma may progress into a persistent vegetative state (PVS). PVS is a condition of partial arousal in which a person may have a low level of consciousness but is still unable to react to external stimuli. In contrast to coma, a person in a PVS retains sleep/wake cycles and may exhibit some key lower brain functions, such as spontaneous movement, opening and moving eyes, and grimacing. Coma or PVS may result from TBI, a nontraumatic insult to the brain (such as a vascular insult, infection, or brain tumor), or a neurodegenerative or metabolic disorder. Medically induced comas are not considered under 11.20 and should be considered under the section pertaining to the underlying reason the coma was medically induced and not under this section.
S. What are motor neuron disorders, other than ALS, and how do we evaluate them under 11.22? Motor neuron disorders such as progressive bulbar palsy, primary lateral sclerosis (PLS), and spinal muscular atrophy (SMA) are progressive neurological disorders that destroy the cells that control voluntary muscle activity, such as walking, breathing, swallowing, and speaking. We evaluate the effects of these disorders on motor functioning, bulbar and neuromuscular functioning, oral communication, or limitations in physical and mental functioning.
T. How do we consider symptoms of fatigue in these listings? Fatigue is one of the most common and limiting symptoms of some neurological disorders, such as multiple sclerosis, post-polio syndrome, and myasthenia gravis. These disorders may result in physical fatigue (lack of muscle strength) or mental fatigue (decreased awareness or attention). When we evaluate your fatigue, we will consider the intensity, persistence, and effects of fatigue on your functioning. This may include information such as the clinical and laboratory data and other objective evidence concerning your neurological deficit, a description of fatigue considered characteristic of your disorder, and information about your functioning. We consider the effects of physical fatigue on your ability to stand up, balance, walk, or perform fine and gross motor movements using the criteria described in 11.00D. We consider the effects of physical and mental fatigue when we evaluate your physical and mental functioning described in 11.00G.
U. How do we evaluate your neurological disorder when it does not meet one of these listings?
1. If your neurological disorder does not meet the criteria of any of these listings, we must also consider whether your impairment(s) meets the criteria of a listing in another body system. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. See §§ 404.1526 and 416.926 of this chapter.
2. If your impairment(s) does not meet or medically equal the criteria of a listing, you may or may not have the residual functional capacity to perform your past relevant work or adjust to other work that exists in significant numbers in the national economy, which we determine at the fourth and, if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter.
3. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
11.01 Category of Impairments, Neurological Disorders11.02 Epilepsy, documented by a detailed description of a typical seizure and characterized by A, B, C, or D:
A. Generalized tonic-clonic seizures (see 11.00H1a), occurring at least once a month for at least 3 consecutive months (see 11.00H4) despite adherence to prescribed treatment (see 11.00C); or
B. Dyscognitive seizures (see 11.00H1b), occurring at least once a week for at least 3 consecutive months (see 11.00H4) despite adherence to prescribed treatment (see 11.00C); or
C. Generalized tonic-clonic seizures (see 11.00H1a), occurring at least once every 2 months for at least 4 consecutive months (see 11.00H4) despite adherence to prescribed treatment (see 11.00C); and a marked limitation in one of the following:
1. Physical functioning (see 11.00G3a); or
2. Understanding, remembering, or applying information (see 11.00G3b(i)); or
3. Interacting with others (see 11.00G3b(ii)); or
4. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
5. Adapting or managing oneself (see 11.00G3b(iv)); or
D. Dyscognitive seizures (see 11.00H1b), occurring at least once every 2 weeks for at least 3 consecutive months (see 11.00H4) despite adherence to prescribed treatment (see 11.00C); and a marked limitation in one of the following:
1. Physical functioning (see 11.00G3a); or
2. Understanding, remembering, or applying information (see 11.00G3b(i)); or
3. Interacting with others (see 11.00G3b(ii)); or
4. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
5. Adapting or managing oneself (see 11.00G3b(iv)).
11.03 [Reserved]
11.04 Vascular insult to the brain, characterized by A, B, or C:
A. Sensory or motor aphasia resulting in ineffective speech or communication (see 11.00E1) persisting for at least 3 consecutive months after the insult; or
B. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities, persisting for at least 3 consecutive months after the insult; or
C. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a) and in one of the following areas of mental functioning, both persisting for at least 3 consecutive months after the insult:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.05 Benign brain tumors, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.06 Parkinsonian syndrome, characterized by A or B despite adherence to prescribed treatment for at least 3 consecutive months (see 11.00C):
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.07 Cerebral palsy, characterized by A, B, or C:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)); or
C. Significant interference in communication due to speech, hearing, or visual deficit (see 11.00E2).
11.08 Spinal cord disorders, characterized by A, B, or C:
A. Complete loss of function, as described in 11.00M2, persisting for 3 consecutive months after the disorder (see 11.00M4); or
B. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities persisting for 3 consecutive months after the disorder (see 11.00M4); or
C. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a) and in one of the following areas of mental functioning, both persisting for 3 consecutive months after the disorder (see 11.00M4):
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.09 Multiple sclerosis, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.10 Amyotrophic lateral sclerosis (ALS) established by clinical and laboratory findings (see 11.00O).
11.11 Post-polio syndrome, characterized by A, B, C, or D:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Unintelligible speech (see 11.00E3); or
C. Bulbar and neuromuscular dysfunction (see 11.00F), resulting in:
1. Acute respiratory failure requiring mechanical ventilation; or
2. Need for supplemental enteral nutrition via a gastrostomy or parenteral nutrition via a central venous catheter; or
D. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.12 Myasthenia gravis, characterized by A, B, or C despite adherence to prescribed treatment for at least 3 months (see 11.00C):
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Bulbar and neuromuscular dysfunction (see 11.00F), resulting in:
1. One myasthenic crisis requiring mechanical ventilation; or
2. Need for supplemental enteral nutrition via a gastrostomy or parenteral nutrition via a central venous catheter; or
C. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.13 Muscular dystrophy, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.14 Peripheral neuropathy, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.15 [Reserved]
11.16 [Reserved]
11.17 Neurodegenerative disorders of the central nervous system, such as Huntington's disease, Friedreich's ataxia, and spinocerebellar degeneration, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.18 Traumatic brain injury, characterized by A or B:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities, persisting for at least 3 consecutive months after the injury; or
B. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following areas of mental functioning, persisting for at least 3 consecutive months after the injury:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
11.19 [Reserved]
11.20 Coma or persistent vegetative state, persisting for at least 1 month.
11.21 [Reserved]
11.22 Motor neuron disorders other than ALS, characterized by A, B, or C:
A. Disorganization of motor function in two extremities (see 11.00D1), resulting in an extreme limitation (see 11.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Bulbar and neuromuscular dysfunction (see 11.00F), resulting in:
1. Acute respiratory failure requiring invasive mechanical ventilation; or
2. Need for supplemental enteral nutrition via a gastrostomy or parenteral nutrition via a central venous catheter; or
C. Marked limitation (see 11.00G2) in physical functioning (see 11.00G3a), and in one of the following:
1. Understanding, remembering, or applying information (see 11.00G3b(i)); or
2. Interacting with others (see 11.00G3b(ii)); or
3. Concentrating, persisting, or maintaining pace (see 11.00G3b(iii)); or
4. Adapting or managing oneself (see 11.00G3b(iv)).
12.00 Mental DisordersA. How are the listings for mental disorders arranged, and what do they require?
1. The listings for mental disorders are arranged in 11 categories: Neurocognitive disorders (12.02); schizophrenia spectrum and other psychotic disorders (12.03); depressive, bipolar and related disorders (12.04); intellectual disorder (12.05); anxiety and obsessive-compulsive disorders (12.06); somatic symptom and related disorders (12.07); personality and impulse-control disorders (12.08); autism spectrum disorder (12.10); neurodevelopmental disorders (12.11); eating disorders (12.13); and trauma- and stressor-related disorders (12.15).
2. Listings 12.07, 12.08, 12.10, 12.11, and 12.13 have two paragraphs, designated A and B; your mental disorder must satisfy the requirements of both paragraphs A and B. Listings 12.02, 12.03, 12.04, 12.06, and 12.15 have three paragraphs, designated A, B, and C; your mental disorder must satisfy the requirements of both paragraphs A and B, or the requirements of both paragraphs A and C. Listing 12.05 has two paragraphs that are unique to that listing (see 12.00A3); your mental disorder must satisfy the requirements of either paragraph A or paragraph B.
a. Paragraph A of each listing (except 12.05) includes the medical criteria that must be present in your medical evidence.
b. Paragraph B of each listing (except 12.05) provides the functional criteria we assess, in conjunction with a rating scale (see 12.00E and 12.00F), to evaluate how your mental disorder limits your functioning. These criteria represent the areas of mental functioning a person uses in a work setting. They are: Understand, remember, or apply information; interact with others; concentrate, persist, or maintain pace; and adapt or manage oneself. We will determine the degree to which your medically determinable mental impairment affects the four areas of mental functioning and your ability to function independently, appropriately, effectively, and on a sustained basis (see §§ 404.1520a(c)(2) and 416.920a(c)(2) of this chapter). To satisfy the paragraph B criteria, your mental disorder must result in “extreme” limitation of one, or “marked” limitation of two, of the four areas of mental functioning. (When we refer to “paragraph B criteria” or “area[s] of mental functioning” in the introductory text of this body system, we mean the criteria in paragraph B of every listing except 12.05.)
c. Paragraph C of listings 12.02, 12.03, 12.04, 12.06, and 12.15 provides the criteria we use to evaluate “serious and persistent mental disorders.” To satisfy the paragraph C criteria, your mental disorder must be “serious and persistent”; that is, there must be a medically documented history of the existence of the disorder over a period of at least 2 years, and evidence that satisfies the criteria in both C1 and C2 (see 12.00G). (When we refer to “paragraph C” or “the paragraph C criteria” in the introductory text of this body system, we mean the criteria in paragraph C of listings 12.02, 12.03, 12.04, 12.06, and 12.15.)
3. Listing 12.05 has two paragraphs, designated A and B, that apply to only intellectual disorder. Each paragraph requires that you have significantly subaverage general intellectual functioning; significant deficits in current adaptive functioning; and evidence that demonstrates or supports (is consistent with) the conclusion that your disorder began prior to age 22.
B. Which mental disorders do we evaluate under each listing category?
1. Neurocognitive disorders (12.02).
a. These disorders are characterized by a clinically significant decline in cognitive functioning. Symptoms and signs may include, but are not limited to, disturbances in memory, executive functioning (that is, higher-level cognitive processes; for example, regulating attention, planning, inhibiting responses, decision-making), visual-spatial functioning, language and speech, perception, insight, judgment, and insensitivity to social standards.
b. Examples of disorders that we evaluate in this category include major neurocognitive disorder; dementia of the Alzheimer type; vascular dementia; dementia due to a medical condition such as a metabolic disease (for example, late-onset Tay-Sachs disease), human immunodeficiency virus infection, vascular malformation, progressive brain tumor, neurological disease (for example, multiple sclerosis, Parkinsonian syndrome, Huntington disease), or traumatic brain injury; or substance-induced cognitive disorder associated with drugs of abuse, medications, or toxins. (We evaluate neurological disorders under that body system (see 11.00). We evaluate cognitive impairments that result from neurological disorders under 12.02 if they do not satisfy the requirements in 11.00 (see 11.00G).)
c. This category does not include the mental disorders that we evaluate under intellectual disorder (12.05), autism spectrum disorder (12.10), and neurodevelopmental disorders (12.11).
2. Schizophrenia spectrum and other psychotic disorders (12.03).
a. These disorders are characterized by delusions, hallucinations, disorganized speech, or grossly disorganized or catatonic behavior, causing a clinically significant decline in functioning. Symptoms and signs may include, but are not limited to, inability to initiate and persist in goal-directed activities, social withdrawal, flat or inappropriate affect, poverty of thought and speech, loss of interest or pleasure, disturbances of mood, odd beliefs and mannerisms, and paranoia.
b. Examples of disorders that we evaluate in this category include schizophrenia, schizoaffective disorder, delusional disorder, and psychotic disorder due to another medical condition.
3. Depressive, bipolar and related disorders (12.04).
a. These disorders are characterized by an irritable, depressed, elevated, or expansive mood, or by a loss of interest or pleasure in all or almost all activities, causing a clinically significant decline in functioning. Symptoms and signs may include, but are not limited to, feelings of hopelessness or guilt, suicidal ideation, a clinically significant change in body weight or appetite, sleep disturbances, an increase or decrease in energy, psychomotor abnormalities, disturbed concentration, pressured speech, grandiosity, reduced impulse control, sadness, euphoria, and social withdrawal.
b. Examples of disorders that we evaluate in this category include bipolar disorders (I or II), cyclothymic disorder, major depressive disorder, persistent depressive disorder (dysthymia), and bipolar or depressive disorder due to another medical condition.
4. Intellectual disorder (12.05).
a. This disorder is characterized by significantly subaverage general intellectual functioning, significant deficits in current adaptive functioning, and manifestation of the disorder before age 22. Signs may include, but are not limited to, poor conceptual, social, or practical skills evident in your adaptive functioning.
b. The disorder that we evaluate in this category may be described in the evidence as intellectual disability, intellectual developmental disorder, or historically used terms such as “mental retardation.”
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (12.02), autism spectrum disorder (12.10), or neurodevelopmental disorders (12.11).
5. Anxiety and obsessive-compulsive disorders (12.06).
a. These disorders are characterized by excessive anxiety, worry, apprehension, and fear, or by avoidance of feelings, thoughts, activities, objects, places, or people. Symptoms and signs may include, but are not limited to, restlessness, difficulty concentrating, hyper-vigilance, muscle tension, sleep disturbance, fatigue, panic attacks, obsessions and compulsions, constant thoughts and fears about safety, and frequent physical complaints.
b. Examples of disorders that we evaluate in this category include social anxiety disorder, panic disorder, generalized anxiety disorder, agoraphobia, and obsessive-compulsive disorder.
c. This category does not include the mental disorders that we evaluate under trauma- and stressor-related disorders (12.15).
6. Somatic symptom and related disorders (12.07).
a. These disorders are characterized by physical symptoms or deficits that are not intentionally produced or feigned, and that, following clinical investigation, cannot be fully explained by a general medical condition, another mental disorder, the direct effects of a substance, or a culturally sanctioned behavior or experience. These disorders may also be characterized by a preoccupation with having or acquiring a serious medical condition that has not been identified or diagnosed. Symptoms and signs may include, but are not limited to, pain and other abnormalities of sensation, gastrointestinal symptoms, fatigue, a high level of anxiety about personal health status, abnormal motor movement, pseudoseizures, and pseudoneurological symptoms, such as blindness or deafness.
b. Examples of disorders that we evaluate in this category include somatic symptom disorder, illness anxiety disorder, and conversion disorder.
7. Personality and impulse-control disorders (12.08).
a. These disorders are characterized by enduring, inflexible, maladaptive, and pervasive patterns of behavior. Onset typically occurs in adolescence or young adulthood. Symptoms and signs may include, but are not limited to, patterns of distrust, suspiciousness, and odd beliefs; social detachment, discomfort, or avoidance; hypersensitivity to negative evaluation; an excessive need to be taken care of; difficulty making independent decisions; a preoccupation with orderliness, perfectionism, and control; and inappropriate, intense, impulsive anger and behavioral expression grossly out of proportion to any external provocation or psychosocial stressors.
b. Examples of disorders that we evaluate in this category include paranoid, schizoid, schizotypal, borderline, avoidant, dependent, obsessive-compulsive personality disorders, and intermittent explosive disorder.
8. Autism spectrum disorder (12.10).
a. These disorders are characterized by qualitative deficits in the development of reciprocal social interaction, verbal and nonverbal communication skills, and symbolic or imaginative activity; restricted repetitive and stereotyped patterns of behavior, interests, and activities; and stagnation of development or loss of acquired skills early in life. Symptoms and signs may include, but are not limited to, abnormalities and unevenness in the development of cognitive skills; unusual responses to sensory stimuli; and behavioral difficulties, including hyperactivity, short attention span, impulsivity, aggressiveness, or self-injurious actions.
b. Examples of disorders that we evaluate in this category include autism spectrum disorder with or without accompanying intellectual impairment, and autism spectrum disorder with or without accompanying language impairment.
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (12.02), intellectual disorder (12.05), and neurodevelopmental disorders (12.11).
9. Neurodevelopmental disorders (12.11).
a. These disorders are characterized by onset during the developmental period, that is, during childhood or adolescence, although sometimes they are not diagnosed until adulthood. Symptoms and signs may include, but are not limited to, underlying abnormalities in cognitive processing (for example, deficits in learning and applying verbal or nonverbal information, visual perception, memory, or a combination of these); deficits in attention or impulse control; low frustration tolerance; excessive or poorly planned motor activity; difficulty with organizing (time, space, materials, or tasks); repeated accidental injury; and deficits in social skills. Symptoms and signs specific to tic disorders include sudden, rapid, recurrent, non-rhythmic, motor movement or vocalization.
b. Examples of disorders that we evaluate in this category include specific learning disorder, borderline intellectual functioning, and tic disorders (such as Tourette syndrome).
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (12.02), autism spectrum disorder (12.10), or personality and impulse-control disorders (12.08).
10. Eating disorders (12.13).
a. These disorders are characterized by disturbances in eating behavior and preoccupation with, and excessive self-evaluation of, body weight and shape. Symptoms and signs may include, but are not limited to, restriction of energy consumption when compared with individual requirements; recurrent episodes of binge eating or behavior intended to prevent weight gain, such as self-induced vomiting, excessive exercise, or misuse of laxatives; mood disturbances, social withdrawal, or irritability; amenorrhea; dental problems; abnormal laboratory findings; and cardiac abnormalities.
b. Examples of disorders that we evaluate in this category include anorexia nervosa, bulimia nervosa, binge-eating disorder, and avoidant/restrictive food disorder.
11. Trauma- and stressor-related disorders (12.15).
a. These disorders are characterized by experiencing or witnessing a traumatic or stressful event, or learning of a traumatic event occurring to a close family member or close friend, and the psychological aftermath of clinically significant effects on functioning. Symptoms and signs may include, but are not limited to, distressing memories, dreams, and flashbacks related to the trauma or stressor; avoidant behavior; diminished interest or participation in significant activities; persistent negative emotional states (for example, fear, anger) or persistent inability to experience positive emotions (for example, satisfaction, affection); anxiety; irritability; aggression; exaggerated startle response; difficulty concentrating; and sleep disturbance.
b. Examples of disorders that we evaluate in this category include posttraumatic stress disorder and other specified trauma- and stressor-related disorders (such as adjustment-like disorders with prolonged duration without prolonged duration of stressor).
c. This category does not include the mental disorders that we evaluate under anxiety and obsessive-compulsive disorders (12.06), and cognitive impairments that result from neurological disorders, such as a traumatic brain injury, which we evaluate under neurocognitive disorders (12.02).
C. What evidence do we need to evaluate your mental disorder?
1. General. We need objective medical evidence from an acceptable medical source to establish that you have a medically determinable mental disorder. We also need evidence to assess the severity of your mental disorder and its effects on your ability to function in a work setting. We will determine the extent and kinds of evidence we need from medical and nonmedical sources based on the individual facts about your disorder. For additional evidence requirements for intellectual disorder (12.05), see 12.00H. For our basic rules on evidence, see §§ 404.1512, 404.1513, 404.1520b, 416.912, 416.913, and 416.920b of this chapter. For our rules on evaluating medical opinions, see §§ 404.1520c, 404.1527, 416.920c, and 416.927 of this chapter. For our rules on evidence about your symptoms, see §§ 404.1529 and 416.929 of this chapter.
2. Evidence from medical sources. We will consider all relevant medical evidence about your disorder from your physician, psychologist, and other medical sources, which include health care providers such as physician assistants, psychiatric nurse practitioners, licensed clinical social workers, and clinical mental health counselors. Evidence from your medical sources may include:
a. Your reported symptoms.
b. Your medical, psychiatric, and psychological history.
c. The results of physical or mental status examinations, structured clinical interviews, psychiatric or psychological rating scales, measures of adaptive functioning, or other clinical findings.
d. Psychological testing, imaging results, or other laboratory findings.
e. Your diagnosis.
f. The type, dosage, and beneficial effects of medications you take.
g. The type, frequency, duration, and beneficial effects of therapy you receive.
h. Side effects of medication or other treatment that limit your ability to function.
i. Your clinical course, including changes in your medication, therapy, or other treatment, and the time required for therapeutic effectiveness.
j. Observations and descriptions of how you function during examinations or therapy.
k. Information about sensory, motor, or speech abnormalities, or about your cultural background (for example, language or customs) that may affect an evaluation of your mental disorder.
l. The expected duration of your symptoms and signs and their effects on your functioning, both currently and in the future.
3. Evidence from you and people who know you. We will consider all relevant evidence about your mental disorder and your daily functioning that we receive from you and from people who know you. We will ask about your symptoms, your daily functioning, and your medical treatment. We will ask for information from third parties who can tell us about your mental disorder, but you must give us permission to do so. This evidence may include information from your family, caregivers, friends, neighbors, clergy, case managers, social workers, shelter staff, or other community support and outreach workers. We will consider whether your statements and the statements from third parties are consistent with the medical and other evidence we have.
4. Evidence from school, vocational training, work, and work-related programs.
a. School. You may have recently attended or may still be attending school, and you may have received or may still be receiving special education services. If so, we will try to obtain information from your school sources when we need it to assess how your mental disorder affects your ability to function. Examples of this information include your Individualized Education Programs (IEPs), your Section 504 plans, comprehensive evaluation reports, school-related therapy progress notes, information from your teachers about how you function in a classroom setting, and information about any special services or accommodations you receive at school.
b. Vocational training, work, and work-related programs. You may have recently participated in or may still be participating in vocational training, work-related programs, or work activity. If so, we will try to obtain information from your training program or your employer when we need it to assess how your mental disorder affects your ability to function. Examples of this information include training or work evaluations, modifications to your work duties or work schedule, and any special supports or accommodations you have required or now require in order to work. If you have worked or are working through a community mental health program, sheltered or supported work program, rehabilitation program, or transitional employment program, we will consider the type and degree of support you have received or are receiving in order to work (see 12.00D).
5. Need for longitudinal evidence.
a. General. Longitudinal medical evidence can help us learn how you function over time, and help us evaluate any variations in the level of your functioning. We will request longitudinal evidence of your mental disorder when your medical providers have records concerning you and your mental disorder over a period of months or perhaps years (see §§ 404.1512(d) and 416.912(d) of this chapter).
b. Non-medical sources of longitudinal evidence. Certain situations, such as chronic homelessness, may make it difficult for you to provide longitudinal medical evidence. If you have a severe mental disorder, you will probably have evidence of its effects on your functioning over time, even if you have not had an ongoing relationship with the medical community or are not currently receiving treatment. For example, family members, friends, neighbors, former employers, social workers, case managers, community support staff, outreach workers, or government agencies may be familiar with your mental health history. We will ask for information from third parties who can tell us about your mental disorder, but you must give us permission to do so.
c. Absence of longitudinal evidence. In the absence of longitudinal evidence, we will use current objective medical evidence and all other relevant evidence available to us in your case record to evaluate your mental disorder. If we purchase a consultative examination to document your disorder, the record will include the results of that examination (see §§ 404.1514 and 416.914 of this chapter). We will take into consideration your medical history, symptoms, clinical and laboratory findings, and medical source opinions. If you do not have longitudinal evidence, the current evidence alone may not be sufficient or appropriate to show that you have a disorder that meets the criteria of one of the mental disorders listings. In that case, we will follow the rules in 12.00J.
6. Evidence of functioning in unfamiliar situations or supportive situations.
a. Unfamiliar situations. We recognize that evidence about your functioning in unfamiliar situations does not necessarily show how you would function on a sustained basis in a work setting. In one-time, time-limited, or other unfamiliar situations, you may function differently than you do in familiar situations. In unfamiliar situations, you may appear more, or less, limited than you do on a daily basis and over time.
b. Supportive situations. Your ability to complete tasks in settings that are highly structured, or that are less demanding or more supportive than typical work settings does not necessarily demonstrate your ability to complete tasks in the context of regular employment during a normal workday or work week.
c. Our assessment. We must assess your ability to complete tasks by evaluating all the evidence, such as reports about your functioning from you and third parties who are familiar with you, with an emphasis on how independently, appropriately, and effectively you are able to complete tasks on a sustained basis.
D. How do we consider psychosocial supports, structured settings, living arrangements, and treatment?
1. General. Psychosocial supports, structured settings, and living arrangements, including assistance from your family or others, may help you by reducing the demands made on you. In addition, treatment you receive may reduce your symptoms and signs and possibly improve your functioning, or may have side effects that limit your functioning. Therefore, when we evaluate the effects of your mental disorder and rate the limitation of your areas of mental functioning, we will consider the kind and extent of supports you receive, the characteristics of any structured setting in which you spend your time, and the effects of any treatment. This evidence may come from reports about your functioning from you or third parties who are familiar with you, and other third-party statements or information. Following are some examples of the supports you may receive:
a. You receive help from family members or other people who monitor your daily activities and help you to function. For example, family members administer your medications, remind you to eat, shop for you and pay your bills, or change their work hours so you are never home alone.
b. You participate in a special education or vocational training program, or a psychosocial rehabilitation day treatment or community support program, where you receive training in daily living and entry-level work skills.
c. You participate in a sheltered, supported, or transitional work program, or in a competitive employment setting with the help of a job coach or supervisor.
d. You receive comprehensive “24/7 wrap-around” mental health services while living in a group home or transitional housing, while participating in a semi-independent living program, or while living in individual housing (for example, your own home or apartment).
e. You live in a hospital or other institution with 24-hour care.
f. You receive assistance from a crisis response team, social workers, or community mental health workers who help you meet your physical needs, and who may also represent you in dealings with government or community social services.
g. You live alone and do not receive any psychosocial support(s); however, you have created a highly structured environment by eliminating all but minimally necessary contact with the world outside your living space.
2. How we consider different levels of support and structure in psychosocial rehabilitation programs.
a. Psychosocial rehabilitation programs are based on your specific needs. Therefore, we cannot make any assumptions about your mental disorder based solely on the fact that you are associated with such a program. We must know the details of the program(s) in which you are involved and the pattern(s) of your involvement over time.
b. The kinds and levels of supports and structures in psychosocial rehabilitation programs typically occur on a scale of “most restrictive” to “least restrictive.” Participation in a psychosocial rehabilitation program at the most restrictive level would suggest greater limitation of your areas of mental functioning than would participation at a less restrictive level. The length of time you spend at different levels in a program also provides information about your functioning. For example, you could begin participation at the most restrictive crisis intervention level but gradually improve to the point of readiness for a lesser level of support and structure and possibly some form of employment.
3. How we consider the help or support you receive.
a. We will consider the complete picture of your daily functioning, including the kinds, extent, and frequency of help and support you receive, when we evaluate your mental disorder and determine whether you are able to use the four areas of mental functioning in a work setting. The fact that you have done, or currently do, some routine activities without help or support does not necessarily mean that you do not have a mental disorder or that you are not disabled. For example, you may be able to take care of your personal needs, cook, shop, pay your bills, live by yourself, and drive a car. You may demonstrate both strengths and deficits in your daily functioning.
b. You may receive various kinds of help and support from others that enable you to do many things that, because of your mental disorder, you might not be able to do independently. Your daily functioning may depend on the special contexts in which you function. For example, you may spend your time among only familiar people or surroundings, in a simple and steady routine or an unchanging environment, or in a highly structured setting. However, this does not necessarily show how you would function in a work setting on a sustained basis, throughout a normal workday and workweek. (See 12.00H for further discussion of these issues regarding significant deficits in adaptive functioning for the purpose of 12.05.)
4. How we consider treatment. We will consider the effect of any treatment on your functioning when we evaluate your mental disorder. Treatment may include medication(s), psychotherapy, or other forms of intervention, which you receive in a doctor's office, during a hospitalization, or in a day program at a hospital or outpatient treatment program. With treatment, you may not only have your symptoms and signs reduced, but may also be able to function in a work setting. However, treatment may not resolve all of the limitations that result from your mental disorder, and the medications you take or other treatment you receive for your disorder may cause side effects that limit your mental or physical functioning. For example, you may experience drowsiness, blunted affect, memory loss, or abnormal involuntary movements.
E. What are the paragraph B criteria?
1. Understand, remember, or apply information (paragraph B1). This area of mental functioning refers to the abilities to learn, recall, and use information to perform work activities. Examples include: Understanding and learning terms, instructions, procedures; following one- or two-step oral instructions to carry out a task; describing work activity to someone else; asking and answering questions and providing explanations; recognizing a mistake and correcting it; identifying and solving problems; sequencing multi-step activities; and using reason and judgment to make work-related decisions. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
2. Interact with others (paragraph B2). This area of mental functioning refers to the abilities to relate to and work with supervisors, co-workers, and the public. Examples include: cooperating with others; asking for help when needed; handling conflicts with others; stating own point of view; initiating or sustaining conversation; understanding and responding to social cues (physical, verbal, emotional); responding to requests, suggestions, criticism, correction, and challenges; and keeping social interactions free of excessive irritability, sensitivity, argumentativeness, or suspiciousness. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
3. Concentrate, persist, or maintain pace (paragraph B3). This area of mental functioning refers to the abilities to focus attention on work activities and stay on task at a sustained rate. Examples include: Initiating and performing a task that you understand and know how to do; working at an appropriate and consistent pace; completing tasks in a timely manner; ignoring or avoiding distractions while working; changing activities or work settings without being disruptive; working close to or with others without interrupting or distracting them; sustaining an ordinary routine and regular attendance at work; and working a full day without needing more than the allotted number or length of rest periods during the day. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
4. Adapt or manage oneself (paragraph B4). This area of mental functioning refers to the abilities to regulate emotions, control behavior, and maintain well-being in a work setting. Examples include: Responding to demands; adapting to changes; managing your psychologically based symptoms; distinguishing between acceptable and unacceptable work performance; setting realistic goals; making plans for yourself independently of others; maintaining personal hygiene and attire appropriate to a work setting; and being aware of normal hazards and taking appropriate precautions. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples.
F. How do we use the paragraph B criteria to evaluate your mental disorder?
1. General. We use the paragraph B criteria, in conjunction with a rating scale (see 12.00F2), to rate the degree of your limitations. We consider only the limitations that result from your mental disorder(s). We will determine whether you are able to use each of the paragraph B areas of mental functioning in a work setting. We will consider, for example, the kind, degree, and frequency of difficulty you would have; whether you could function without extra help, structure, or supervision; and whether you would require special conditions with regard to activities or other people (see 12.00D).
2. The five-point rating scale. We evaluate the effects of your mental disorder on each of the four areas of mental functioning based on a five-point rating scale consisting of none, mild, moderate, marked, and extreme limitation. To satisfy the paragraph B criteria, your mental disorder must result in extreme limitation of one, or marked limitation of two, paragraph B areas of mental functioning. Under these listings, the five rating points are defined as follows:
a. No limitation (or none). You are able to function in this area independently, appropriately, effectively, and on a sustained basis.
b. Mild limitation. Your functioning in this area independently, appropriately, effectively, and on a sustained basis is slightly limited.
c. Moderate limitation. Your functioning in this area independently, appropriately, effectively, and on a sustained basis is fair.
d. Marked limitation. Your functioning in this area independently, appropriately, effectively, and on a sustained basis is seriously limited.
e. Extreme limitation. You are not able to function in this area independently, appropriately, effectively, and on a sustained basis.
3. Rating the limitations of your areas of mental functioning.
a. General. We use all of the relevant medical and non-medical evidence in your case record to evaluate your mental disorder: The symptoms and signs of your disorder, the reported limitations in your activities, and any help and support you receive that is necessary for you to function. The medical evidence may include descriptors regarding the diagnostic stage or level of your disorder, such as “mild” or “moderate.” Clinicians may use these terms to characterize your medical condition. However, these terms will not always be the same as the degree of your limitation in a paragraph B area of mental functioning.
b. Areas of mental functioning in daily activities. You use the same four areas of mental functioning in daily activities at home and in the community that you would use to function at work. With respect to a particular task or activity, you may have trouble using one or more of the areas. For example, you may have difficulty understanding and remembering what to do; or concentrating and staying on task long enough to do it; or engaging in the task or activity with other people; or trying to do the task without becoming frustrated and losing self-control. Information about your daily functioning can help us understand whether your mental disorder limits one or more of these areas; and, if so, whether it also affects your ability to function in a work setting.
c. Areas of mental functioning in work settings. If you have difficulty using an area of mental functioning from day-to-day at home or in your community, you may also have difficulty using that area to function in a work setting. On the other hand, if you are able to use an area of mental functioning at home or in your community, we will not necessarily assume that you would also be able to use that area to function in a work setting where the demands and stressors differ from those at home. We will consider all evidence about your mental disorder and daily functioning before we reach a conclusion about your ability to work.
d. Overall effect of limitations. Limitation of an area of mental functioning reflects the overall degree to which your mental disorder interferes with that area. The degree of limitation is how we document our assessment of your limitation when using the area of mental functioning independently, appropriately, effectively, and on a sustained basis. It does not necessarily reflect a specific type or number of activities, including activities of daily living, that you have difficulty doing. In addition, no single piece of information (including test results) can establish the degree of limitation of an area of mental functioning.
e. Effects of support, supervision, structure on functioning. The degree of limitation of an area of mental functioning also reflects the kind and extent of supports or supervision you receive and the characteristics of any structured setting where you spend your time, which enable you to function. The more extensive the support you need from others or the more structured the setting you need in order to function, the more limited we will find you to be (see 12.00D).
f. Specific instructions for paragraphs B1, B3, and B4. For paragraphs B1, B3, and B4, the greatest degree of limitation of any part of the area of mental functioning directs the rating of limitation of that whole area of mental functioning.
(i) To do a work-related task, you must be able to understand and remember and apply information required by the task. Similarly, you must be able to concentrate and persist and maintain pace in order to complete the task, and adapt and manage yourself in the workplace. Limitation in any one of these parts (understand or remember or apply; concentrate or persist or maintain pace; adapt or manage oneself) may prevent you from completing a work-related task.
(ii) We will document the rating of limitation of the whole area of mental functioning, not each individual part. We will not add ratings of the parts together. For example, with respect to paragraph B3, if you have marked limitation in maintaining pace, and mild or moderate limitations in concentrating and persisting, we will find that you have marked limitation in the whole paragraph B3 area of mental functioning.
(iii) Marked limitation in more than one part of the same paragraph B area of mental functioning does not satisfy the requirement to have marked limitation in two paragraph B areas of mental functioning.
4. How we evaluate mental disorders involving exacerbations and remissions.
a. When we evaluate the effects of your mental disorder, we will consider how often you have exacerbations and remissions, how long they last, what causes your mental disorder to worsen or improve, and any other relevant information. We will assess any limitation of the affected paragraph B area(s) of mental functioning using the rating scale for the paragraph B criteria. We will consider whether you can use the area of mental functioning on a regular and continuing basis (8 hours a day, 5 days a week, or an equivalent work schedule). We will not find that you are able to work solely because you have a period(s) of improvement (remission), or that you are disabled solely because you have a period of worsening (exacerbation), of your mental disorder.
b. If you have a mental disorder involving exacerbations and remissions, you may be able to use the four areas of mental functioning to work for a few weeks or months. Recurrence or worsening of symptoms and signs, however, can interfere enough to render you unable to sustain the work.
G. What are the paragraph C criteria, and how do we use them to evaluate your mental disorder?
1. General. The paragraph C criteria are an alternative to the paragraph B criteria under listings 12.02, 12.03, 12.04, 12.06, and 12.15. We use the paragraph C criteria to evaluate mental disorders that are “serious and persistent.” In the paragraph C criteria, we recognize that mental health interventions may control the more obvious symptoms and signs of your mental disorder.
2. Paragraph C criteria.
a. We find a mental disorder to be “serious and persistent” when there is a medically documented history of the existence of the mental disorder in the listing category over a period of at least 2 years, and evidence shows that your disorder satisfies both C1 and C2.
b. The criterion in C1 is satisfied when the evidence shows that you rely, on an ongoing basis, upon medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s), to diminish the symptoms and signs of your mental disorder (see 12.00D). We consider that you receive ongoing medical treatment when the medical evidence establishes that you obtain medical treatment with a frequency consistent with accepted medical practice for the type of treatment or evaluation required for your medical condition. We will consider periods of inconsistent treatment or lack of compliance with treatment that may result from your mental disorder. If the evidence indicates that the inconsistent treatment or lack of compliance is a feature of your mental disorder, and it has led to an exacerbation of your symptoms and signs, we will not use it as evidence to support a finding that you have not received ongoing medical treatment as required by this paragraph.
c. The criterion in C2 is satisfied when the evidence shows that, despite your diminished symptoms and signs, you have achieved only marginal adjustment. “Marginal adjustment” means that your adaptation to the requirements of daily life is fragile; that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life. We will consider that you have achieved only marginal adjustment when the evidence shows that changes or increased demands have led to exacerbation of your symptoms and signs and to deterioration in your functioning; for example, you have become unable to function outside of your home or a more restrictive setting, without substantial psychosocial supports (see 12.00D). Such deterioration may have necessitated a significant change in medication or other treatment. Similarly, because of the nature of your mental disorder, evidence may document episodes of deterioration that have required you to be hospitalized or absent from work, making it difficult for you to sustain work activity over time.
H. How do we document and evaluate intellectual disorder under 12.05?
1. General. Listing 12.05 is based on the three elements that characterize intellectual disorder: Significantly subaverage general intellectual functioning; significant deficits in current adaptive functioning; and the disorder manifested before age 22.
2. Establishing significantly subaverage general intellectual functioning.
a. Definition. Intellectual functioning refers to the general mental capacity to learn, reason, plan, solve problems, and perform other cognitive functions. Under 12.05A, we identify significantly subaverage general intellectual functioning by the cognitive inability to function at a level required to participate in standardized intelligence testing. Our findings under 12.05A are based on evidence from an acceptable medical source. Under 12.05B, we identify significantly subaverage general intellectual functioning by an IQ score(s) on an individually administered standardized test of general intelligence that meets program requirements and has a mean of 100 and a standard deviation of 15. A qualified specialist (see 12.00H2c) must administer the standardized intelligence testing.
b. Psychometric standards. We will find standardized intelligence test results usable for the purposes of 12.05B1 when the measure employed meets contemporary psychometric standards for validity, reliability, normative data, and scope of measurement; and a qualified specialist has individually administered the test according to all pre-requisite testing conditions.
c. Qualified specialist. A “qualified specialist” is currently licensed or certified at the independent level of practice in the State where the test was performed, and has the training and experience to administer, score, and interpret intelligence tests. If a psychological assistant or paraprofessional administered the test, a supervisory qualified specialist must interpret the test findings and co-sign the examination report.
d. Responsibility for conclusions based on testing. We generally presume that your obtained IQ score(s) is an accurate reflection of your general intellectual functioning, unless evidence in the record suggests otherwise. Examples of this evidence include: a statement from the test administrator indicating that your obtained score is not an accurate reflection of your general intellectual functioning, prior or internally inconsistent IQ scores, or information about your daily functioning. Only qualified specialists, Federal and State agency medical and psychological consultants, and other contracted medical and psychological experts may conclude that your obtained IQ score(s) is not an accurate reflection of your general intellectual functioning. This conclusion must be well supported by appropriate clinical and laboratory diagnostic techniques and must be based on relevant evidence in the case record, such as:
(i) The data obtained in testing;
(ii) Your developmental history, including when your signs and symptoms began;
(iii) Information about how you function on a daily basis in a variety of settings; and
(iv) Clinical observations made during the testing period, such as your ability to sustain attention, concentration, and effort; to relate appropriately to the examiner; and to perform tasks independently without prompts or reminders.
3. Establishing significant deficits in adaptive functioning.
a. Definition. Adaptive functioning refers to how you learn and use conceptual, social, and practical skills in dealing with common life demands. It is your typical functioning at home and in the community, alone or among others. Under 12.05A, we identify significant deficits in adaptive functioning based on your dependence on others to care for your personal needs, such as eating and bathing. We will base our conclusions about your adaptive functioning on evidence from a variety of sources (see 12.00H3b) and not on your statements alone. Under 12.05B2, we identify significant deficits in adaptive functioning based on whether there is extreme limitation of one, or marked limitation of two, of the paragraph B criteria (see 12.00E; 12.00F).
b. Evidence. Evidence about your adaptive functioning may come from:
(i) Medical sources, including their clinical observations;
(ii) Standardized tests of adaptive functioning (see 12.00H3c);
(iii) Third party information, such as a report of your functioning from a family member or friend;
(iv) School records, if you were in school recently;
(v) Reports from employers or supervisors; and
(vi) Your own statements about how you handle all of your daily activities.
c. Standardized tests of adaptive functioning. We do not require the results of an individually administered standardized test of adaptive functioning. If your case record includes these test results, we will consider the results along with all other relevant evidence; however, we will use the guidelines in 12.00E and F to evaluate and determine the degree of your deficits in adaptive functioning, as required under 12.05B2.
d. How we consider common everyday activities.
(i) The fact that you engage in common everyday activities, such as caring for your personal needs, preparing simple meals, or driving a car, will not always mean that you do not have deficits in adaptive functioning as required by 12.05B2. You may demonstrate both strengths and deficits in your adaptive functioning. However, a lack of deficits in one area does not negate the presence of deficits in another area. When we assess your adaptive functioning, we will consider all of your activities and your performance of them.
(ii) Our conclusions about your adaptive functioning rest on whether you do your daily activities independently, appropriately, effectively, and on a sustained basis. If you receive help in performing your activities, we need to know the kind, extent, and frequency of help you receive in order to perform them. We will not assume that your ability to do some common everyday activities, or to do some things without help or support, demonstrates that your mental disorder does not meet the requirements of 12.05B2. (See 12.00D regarding the factors we consider when we evaluate your functioning, including how we consider any help or support you receive.)
e. How we consider work activity. The fact that you have engaged in work activity, or that you work intermittently or steadily in a job commensurate with your abilities, will not always mean that you do not have deficits in adaptive functioning as required by 12.05B2. When you have engaged in work activity, we need complete information about the work, and about your functioning in the work activity and work setting, before we reach any conclusions about your adaptive functioning. We will consider all factors involved in your work history before concluding whether your impairment satisfies the criteria for intellectual disorder under 12.05B. We will consider your prior and current work history, if any, and various other factors influencing how you function. For example, we consider whether the work was in a supported setting, whether you required more supervision than other employees, how your job duties compared to others in the same job, how much time it took you to learn the job duties, and the reason the work ended, if applicable.
4. Establishing that the disorder began before age 22. We require evidence that demonstrates or supports (is consistent with) the conclusion that your mental disorder began prior to age 22. We do not require evidence that your impairment met all of the requirements of 12.05A or 12.05B prior to age 22. Also, we do not require you to have met our statutory definition of disability prior to age 22. When we do not have evidence that was recorded before you attained age 22, we need evidence about your current intellectual and adaptive functioning and the history of your disorder that supports the conclusion that the disorder began before you attained age 22. Examples of evidence that can demonstrate or support this conclusion include:
a. Tests of intelligence or adaptive functioning;
b. School records indicating a history of special education services based on your intellectual functioning;
c. An Individualized Education Program (IEP), including your transition plan;
d. Reports of your academic performance and functioning at school;
e. Medical treatment records;
f. Interviews or reports from employers;
g. Statements from a supervisor in a group home or a sheltered workshop; and
h. Statements from people who have known you and can tell us about your functioning in the past and currently.
I. How do we evaluate substance use disorders? If we find that you are disabled and there is medical evidence in your case record establishing that you have a substance use disorder, we will determine whether your substance use disorder is a contributing factor material to the determination of disability (see §§ 404.1535 and 416.935 of this chapter).
J. How do we evaluate mental disorders that do not meet one of the mental disorders listings?
1. These listings include only examples of mental disorders that we consider serious enough to prevent you from doing any gainful activity. If your severe mental disorder does not meet the criteria of any of these listings, we will consider whether you have an impairment(s) that meets the criteria of a listing in another body system. You may have another impairment(s) that is secondary to your mental disorder. For example, if you have an eating disorder and develop a cardiovascular impairment because of it, we will evaluate your cardiovascular impairment under the listings for the cardiovascular body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing (see §§ 404.1526 and 416.926 of this chapter).
3. If your impairment(s) does not meet or medically equal a listing, we will assess your residual functional capacity for engaging in substantial gainful activity (see §§ 404.1545 and 416.945 of this chapter). When we assess your residual functional capacity, we consider all of your impairment-related mental and physical limitations. For example, the side effects of some medications may reduce your general alertness, concentration, or physical stamina, affecting your residual functional capacity for non-exertional or exertional work activities. Once we have determined your residual functional capacity, we proceed to the fourth, and if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
12.01 Category of Impairments, Mental Disorders12.02 Neurocognitive disorders (see 12.00B1), satisfied by A and B, or A and C:
A. Medical documentation of a significant cognitive decline from a prior level of functioning in one or more of the cognitive areas:
1. Complex attention;
2. Executive function;
3. Learning and memory;
4. Language;
5. Perceptual-motor; or
6. Social cognition.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 12.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 12.00G2c).
12.03 Schizophrenia spectrum and other psychotic disorders (see 12.00B2), satisfied by A and B, or A and C:
A. Medical documentation of one or more of the following:
1. Delusions or hallucinations;
2. Disorganized thinking (speech); or
3. Grossly disorganized behavior or catatonia.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 12.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 12.00G2c).
12.04 Depressive, bipolar and related disorders (see 12.00B3), satisfied by A and B, or A and C:
A. Medical documentation of the requirements of paragraph 1 or 2:
1. Depressive disorder, characterized by five or more of the following:
a. Depressed mood;
b. Diminished interest in almost all activities;
c. Appetite disturbance with change in weight;
d. Sleep disturbance;
e. Observable psychomotor agitation or retardation;
f. Decreased energy;
g. Feelings of guilt or worthlessness;
h. Difficulty concentrating or thinking; or
i. Thoughts of death or suicide.
2. Bipolar disorder, characterized by three or more of the following:
a. Pressured speech;
b. Flight of ideas;
c. Inflated self-esteem;
d. Decreased need for sleep;
e. Distractibility;
f. Involvement in activities that have a high probability of painful consequences that are not recognized; or
g. Increase in goal-directed activity or psychomotor agitation.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 12.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 12.00G2c).
12.05 Intellectual disorder (see 12.00B4), satisfied by A or B:
A. Satisfied by 1, 2, and 3 (see 12.00H):
1. Significantly subaverage general intellectual functioning evident in your cognitive inability to function at a level required to participate in standardized testing of intellectual functioning; and
2. Significant deficits in adaptive functioning currently manifested by your dependence upon others for personal needs (for example, toileting, eating, dressing, or bathing); and
3. The evidence about your current intellectual and adaptive functioning and about the history of your disorder demonstrates or supports the conclusion that the disorder began prior to your attainment of age 22.
ORB. Satisfied by 1, 2, and 3 (see 12.00H):
1. Significantly subaverage general intellectual functioning evidenced by a or b:
a. A full scale (or comparable) IQ score of 70 or below on an individually administered standardized test of general intelligence; or
b. A full scale (or comparable) IQ score of 71-75 accompanied by a verbal or performance IQ score (or comparable part score) of 70 or below on an individually administered standardized test of general intelligence; and
2. Significant deficits in adaptive functioning currently manifested by extreme limitation of one, or marked limitation of two, of the following areas of mental functioning:
a. Understand, remember, or apply information (see 12.00E1); or
b. Interact with others (see 12.00E2); or
c. Concentrate, persist, or maintain pace (see 12.00E3); or
d. Adapt or manage oneself (see 12.00E4); and
3. The evidence about your current intellectual and adaptive functioning and about the history of your disorder demonstrates or supports the conclusion that the disorder began prior to your attainment of age 22.
12.06 Anxiety and obsessive-compulsive disorders (see 12.00B5), satisfied by A and B, or A and C:
A. Medical documentation of the requirements of paragraph 1, 2, or 3:
1. Anxiety disorder, characterized by three or more of the following;
a. Restlessness;
b. Easily fatigued;
c. Difficulty concentrating;
d. Irritability;
e. Muscle tension; or
f. Sleep disturbance.
2. Panic disorder or agoraphobia, characterized by one or both:
a. Panic attacks followed by a persistent concern or worry about additional panic attacks or their consequences; or
b. Disproportionate fear or anxiety about at least two different situations (for example, using public transportation, being in a crowd, being in a line, being outside of your home, being in open spaces).
3. Obsessive-compulsive disorder, characterized by one or both:
a. Involuntary, time-consuming preoccupation with intrusive, unwanted thoughts; or
b. Repetitive behaviors aimed at reducing anxiety.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 12.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 12.00G2c).
12.07 Somatic symptom and related disorders (see 12.00B6), satisfied by A and B:
A. Medical documentation of one or more of the following:
1. Symptoms of altered voluntary motor or sensory function that are not better explained by another medical or mental disorder;
2. One or more somatic symptoms that are distressing, with excessive thoughts, feelings, or behaviors related to the symptoms; or
3. Preoccupation with having or acquiring a serious illness without significant symptoms present.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
12.08 Personality and impulse-control disorders (see 12.00B7), satisfied by A and B:
A. Medical documentation of a pervasive pattern of one or more of the following:
1. Distrust and suspiciousness of others;
2. Detachment from social relationships;
3. Disregard for and violation of the rights of others;
4. Instability of interpersonal relationships;
5. Excessive emotionality and attention seeking;
6. Feelings of inadequacy;
7. Excessive need to be taken care of;
8. Preoccupation with perfectionism and orderliness; or
9. Recurrent, impulsive, aggressive behavioral outbursts.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
12.09 [Reserved]
12.10 Autism spectrum disorder (see 12.00B8), satisfied by A and B:
A. Medical documentation of both of the following:
1. Qualitative deficits in verbal communication, nonverbal communication, and social interaction; and
2. Significantly restricted, repetitive patterns of behavior, interests, or activities.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
12.11 Neurodevelopmental disorders (see 12.00B9), satisfied by A and B:
A. Medical documentation of the requirements of paragraph 1, 2, or 3:
1. One or both of the following:
a. Frequent distractibility, difficulty sustaining attention, and difficulty organizing tasks; or
b. Hyperactive and impulsive behavior (for example, difficulty remaining seated, talking excessively, difficulty waiting, appearing restless, or behaving as if being “driven by a motor”).
2. Significant difficulties learning and using academic skills; or
3. Recurrent motor movement or vocalization.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
12.12 [Reserved]
12.13 Eating disorders (see 12.00B10), satisfied by A and B:
A. Medical documentation of a persistent alteration in eating or eating-related behavior that results in a change in consumption or absorption of food and that significantly impairs physical or psychological health.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
12.15 Trauma- and stressor-related disorders (see 12.00B11), satisfied by A and B, or A and C:
A. Medical documentation of all of the following:
1. Exposure to actual or threatened death, serious injury, or violence;
2. Subsequent involuntary re-experiencing of the traumatic event (for example, intrusive memories, dreams, or flashbacks);
3. Avoidance of external reminders of the event;
4. Disturbance in mood and behavior; and
5. Increases in arousal and reactivity (for example, exaggerated startle response, sleep disturbance).
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 12.00F):
1. Understand, remember, or apply information (see 12.00E1).
2. Interact with others (see 12.00E2).
3. Concentrate, persist, or maintain pace (see 12.00E3).
4. Adapt or manage oneself (see 12.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 12.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 12.00G2c).
13.00 Cancer (Malignant Neoplastic Diseases)A. What impairments do these listings cover? We use these listings to evaluate all cancers (malignant neoplastic diseases) except certain cancers associated with human immunodeficiency virus (HIV) infection. We use the criteria in 14.11B to evaluate primary central nervous system lymphoma, 14.11C to evaluate primary effusion lymphoma, and 14.11E to evaluate pulmonary Kaposi sarcoma if you also have HIV infection. We evaluate all other cancers associated with HIV infection, for example, Hodgkin lymphoma or non-pulmonary Kaposi sarcoma, under this body system or under 14.11F-I in the immune system disorders body system.
B. What do we consider when we evaluate cancer under these listings? We will consider factors including:
1. Origin of the cancer.
2. Extent of involvement.
3. Duration, frequency, and response to anticancer therapy.
4. Effects of any post-therapeutic residuals.
C. How do we apply these listings? We apply the criteria in a specific listing to a cancer originating from that specific site.
D. What evidence do we need?
1. We need medical evidence that specifies the type, extent, and site of the primary, recurrent, or metastatic lesion. When the primary site cannot be identified, we will use evidence documenting the site(s) of metastasis to evaluate the impairment under 13.27.
2. For operative procedures, including a biopsy or a needle aspiration, we generally need a copy of both the:
a. Operative note, and
b. Pathology report.
3. When we cannot get these documents, we will accept the summary of hospitalization(s) or other medical reports. This evidence should include details of the findings at surgery and, whenever appropriate, the pathological findings.
4. In some situations, we may also need evidence about recurrence, persistence, or progression of the cancer, the response to therapy, and any significant residuals. (See 13.00G.)
E. When do we need longitudinal evidence?
1. Cancer with distant metastases. We generally do not need longitudinal evidence for cancer that has metastasized beyond the regional lymph nodes because this cancer usually meets the requirements of a listing. Exceptions are for cancer with distant metastases that we expect to respond to anticancer therapy. For these exceptions, we usually need a longitudinal record of 3 months after therapy starts to determine whether the therapy achieved its intended effect, and whether this effect is likely to persist.
2. Other cancers. When there are no distant metastases, many of the listings require that we consider your response to initial anticancer therapy; that is, the initial planned treatment regimen. This therapy may consist of a single modality or a combination of modalities; that is, multimodal therapy. (See 13.00I4.)
3. Types of treatment.
a. Whenever the initial planned therapy is a single modality, enough time must pass to allow a determination about whether the therapy will achieve its intended effect. If the treatment fails, the failure often happens within 6 months after treatment starts, and there will often be a change in the treatment regimen.
b. Whenever the initial planned therapy is multimodal, we usually cannot make a determination about the effectiveness of the therapy until we can determine the effects of all the planned modalities. In some cases, we may need to defer adjudication until we can assess the effectiveness of therapy. However, we do not need to defer adjudication to determine whether the therapy will achieve its intended effect if we can make a fully favorable determination or decision based on the length and effects of therapy, or the residuals of the cancer or therapy (see 13.00G).
c. We need evidence under 13.02E, 13.11D, and 13.14C to establish that your treating source initiated multimodal anticancer therapy. We do not need to make a determination about the length or effectiveness of your therapy. Multimodal therapy has been initiated, and satisfies the requirements in 13.02E, 13.11D, and 13.14C, when your treating source starts the first modality. We may defer adjudication if your treating source plans multimodal therapy and has not yet initiated it.
F. How do we evaluate impairments that do not meet one of the cancer listings?
1. These listings are only examples of cancer that we consider severe enough to prevent you from doing any gainful activity. If your severe impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that meets the criteria of a listing in another body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926 of this chapter.) If your impairment(s) does not meet or medically equal a listing, you may or may not have the residual functional capacity to engage in substantial gainful activity. In that situation, we proceed to the fourth, and, if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920 of this chapter. We use the rules in §§ 404.1594 and 416.994 of this chapter, as appropriate, when we decide whether you continue to be disabled.
G. How do we consider the effects of anticancer therapy?
1. How we consider the effects of anticancer therapy under the listings. In many cases, cancers meet listing criteria only if the therapy is not effective and the cancer persists, progresses, or recurs. However, as explained in the following paragraphs, we will not delay adjudication if we can make a fully favorable determination or decision based on the evidence in the case record.
2. Effects can vary widely.
a. We consider each case on an individual basis because the therapy and its toxicity may vary widely. We will request a specific description of the therapy, including these items:
i. Drugs given.
ii. Dosage.
iii. Frequency of drug administration.
iv. Plans for continued drug administration.
v. Extent of surgery.
vi. Schedule and fields of radiation therapy.
b. We will also request a description of the complications or adverse effects of therapy, such as the following:
i. Continuing gastrointestinal symptoms.
ii. Persistent weakness.
iii. Neurological complications.
iv. Cardiovascular complications.
v. Reactive mental disorders.
3. Effects of therapy may change. The severity of the adverse effects of anticancer therapy may change during treatment; therefore, enough time must pass to allow us to evaluate the therapy's effect. The residual effects of treatment are temporary in most instances; however, on occasion, the effects may be disabling for a consecutive period of at least 12 months. In some situations, very serious adverse effects may interrupt and prolong multimodal anticancer therapy for a continuous period of almost 12 months. In these situations, we may determine there is an expectation that your impairment will preclude you from engaging in any gainful activity for at least 12 months.
4. When the initial anticancer therapy is effective. We evaluate any post-therapeutic residual impairment(s) not included in these listings under the criteria for the affected body system. We must consider any complications of therapy. When the residual impairment(s) does not meet or medically equal a listing, we must consider its effect on your ability to do substantial gainful activity.
H. How long do we consider your impairment to be disabling?
1. In some listings, we specify that we will consider your impairment to be disabling until a particular point in time (for example, until at least 12 months from the date of transplantation). We may consider your impairment to be disabling beyond this point when the medical and other evidence justifies it.
2. When a listing does not contain such a specification, we will consider an impairment(s) that meets or medically equals a listing in this body system to be disabling until at least 3 years after onset of complete remission. When the impairment(s) has been in complete remission for at least 3 years, that is, the original tumor or a recurrence (or relapse) and any metastases have not been evident for at least 3 years, the impairment(s) will no longer meet or medically equal the criteria of a listing in this body system.
3. Following the appropriate period, we will consider any residuals, including residuals of the cancer or therapy (see 13.00G), in determining whether you are disabled. If you have a recurrence or relapse of your cancer, your impairment may meet or medically equal one of the listings in this body system again.
I. What do we mean by the following terms?
1. Anticancer therapy means surgery, radiation, chemotherapy, hormones, immunotherapy, or bone marrow or stem cell transplantation. When we refer to surgery as an anticancer treatment, we mean surgical excision for treatment, not for diagnostic purposes.
2. Inoperable means surgery is thought to be of no therapeutic value or the surgery cannot be performed; for example, when you cannot tolerate anesthesia or surgery because of another impairment(s), or you have a cancer that is too large or that has invaded crucial structures. This term does not include situations in which your cancer could have been surgically removed but another method of treatment was chosen; for example, an attempt at organ preservation. Your physician may determine whether the cancer is inoperable before or after you receive neoadjuvant therapy. Neoadjuvant therapy is anticancer therapy, such as chemotherapy or radiation, given before surgery in order to reduce the size of the cancer.
3. Metastases means the spread of cancer cells by blood, lymph, or other body fluid. This term does not include the spread of cancer cells by direct extension of the cancer to other tissues or organs.
4. Multimodal therapy means anticancer therapy that is a combination of at least two types of treatment given in close proximity as a unified whole and usually planned before any treatment has begun. There are three types of treatment modalities: surgery, radiation, and systemic drug therapy (chemotherapy, hormone therapy, and immunotherapy or biological modifier therapy). Examples of multimodal therapy include:
a. Surgery followed by chemotherapy or radiation.
b. Chemotherapy followed by surgery.
c. Chemotherapy and concurrent radiation.
5. Persistent means the planned initial anticancer therapy failed to achieve a complete remission of your cancer; that is, your cancer is evident, even if smaller, after the therapy has ended.
6. Progressive means the cancer becomes more extensive after treatment; that is, there is evidence that your cancer is growing after you have completed at least half of your planned initial anticancer therapy.
7. Recurrent or relapse means the cancer that was in complete remission or entirely removed by surgery has returned.
8. Unresectable means surgery or surgeries did not completely remove the cancer. This term includes situations in which your cancer is incompletely resected or the surgical margins are positive. It does not include situations in which there is a finding of a positive margin(s) if additional surgery obtains a margin(s) that is clear. It also does not include situations in which the cancer is completely resected but you are receiving adjuvant therapy. Adjuvant therapy is anticancer therapy, such as chemotherapy or radiation, given after surgery in order to eliminate any remaining cancer cells or lessen the chance of recurrence.
J. Can we establish the existence of a disabling impairment prior to the date of the evidence that shows the cancer satisfies the criteria of a listing? Yes. We will consider factors such as:
1. The type of cancer and its location.
2. The extent of involvement when the cancer was first demonstrated.
3. Your symptoms.
K. How do we evaluate specific cancers?
1. Lymphoma.
a. Many indolent (non-aggressive) lymphomas are controlled by well-tolerated treatment modalities, although the lymphomas may produce intermittent symptoms and signs. We may defer adjudicating these cases for an appropriate period after therapy is initiated to determine whether the therapy will achieve its intended effect, which is usually to stabilize the disease process. (See 13.00E3.) Once your disease stabilizes, we will assess severity based on the extent of involvement of other organ systems and residuals from therapy.
b. A change in therapy for indolent lymphomas is usually an indicator that the therapy is not achieving its intended effect. However, your impairment will not meet the requirements of 13.05A2 if your therapy is changed solely because you or your physician chooses to change it and not because of a failure to achieve stability.
c. We consider Hodgkin lymphoma that recurs more than 12 months after completing initial anticancer therapy to be a new disease rather than a recurrence.
2. Leukemia.
a. Acute leukemia. The initial diagnosis of acute leukemia, including the accelerated or blast phase of chronic myelogenous (granulocytic) leukemia, is based on definitive bone marrow examination. Additional diagnostic information is based on chromosomal analysis, cytochemical and surface marker studies on the abnormal cells, or other methods consistent with the prevailing state of medical knowledge and clinical practice. Recurrent disease must be documented by peripheral blood, bone marrow, or cerebrospinal fluid examination, or by testicular biopsy. The initial and follow-up pathology reports should be included.
b. Chronic myelogenous leukemia (CML). We need a diagnosis of CML based on documented granulocytosis, including immature forms such as differentiated or undifferentiated myelocytes and myeloblasts, and a chromosomal analysis that demonstrates the Philadelphia chromosome. In the absence of a chromosomal analysis, or if the Philadelphia chromosome is not present, the diagnosis may be made by other methods consistent with the prevailing state of medical knowledge and clinical practice. The requirement for CML in the accelerated or blast phase is met in 13.06B if laboratory findings show the proportion of blast (immature) cells in the peripheral blood or bone marrow is 10 percent or greater.
c. Chronic lymphocytic leukemia.
i. We require the diagnosis of chronic lymphocytic leukemia (CLL) to be documented by evidence of a chronic lymphocytosis of at least 10,000 cells/mm 3 for 3 months or longer, or other acceptable diagnostic techniques consistent with the prevailing state of medical knowledge and clinical practice.
ii. We evaluate the complications and residual impairment(s) from CLL under the appropriate listings, such as 13.05A2 or the hematological listings (7.00).
d. Elevated white cell count. In cases of chronic leukemia (either myelogenous or lymphocytic), an elevated white cell count, in itself, is not a factor in determining the severity of the impairment.
3. Macroglobulinemia or heavy chain disease. We require the diagnosis of these diseases to be confirmed by protein electrophoresis or immunoelectrophoresis. We evaluate the resulting impairment(s) under the appropriate listings, such as 13.05A2 or the hematological listings (7.00).
4. Primary breast cancer.
a. We evaluate bilateral primary breast cancer (synchronous or metachronous) under 13.10A, which covers local primary disease, and not as a primary disease that has metastasized.
b. We evaluate secondary lymphedema that results from anticancer therapy for breast cancer under 13.10E if the lymphedema is treated by surgery to salvage or restore the functioning of an upper extremity. Secondary lymphedema is edema that results from obstruction or destruction of normal lymphatic channels. We may not restrict our determination of the onset of disability to the date of the surgery; we may establish an earlier onset date of disability if the evidence in your case record supports such a finding.
5. Carcinoma-in-situ. Carcinoma-in-situ, or preinvasive carcinoma, usually responds to treatment. When we use the term “carcinoma” in these listings, it does not include carcinoma-in-situ.
6. Primary central nervous system (CNS) cancers. We use the criteria in 13.13 to evaluate cancers that originate within the CNS (that is, brain and spinal cord cancers).
a. The CNS cancers listed in 13.13A1 are highly malignant and respond poorly to treatment, and therefore we do not require additional criteria to evaluate them. We do not list pituitary gland cancer (for example, pituitary gland carcinoma) in 13.13A1, although this CNS cancer is highly malignant and responds poorly to treatment. We evaluate pituitary gland cancer under 13.13A1 and do not require additional criteria to evaluate it.
b. We consider a CNS tumor to be malignant if it is classified as Grade II, Grade III, or Grade IV under the World Health Organization (WHO) classification of tumors of the CNS (WHO Classification of Tumours of the Central Nervous System, 2007).
c. We evaluate benign (for example, WHO Grade I) CNS tumors under 11.05. We evaluate metastasized CNS cancers from non-CNS sites under the primary cancers (see 13.00C). We evaluate any complications of CNS cancers, such as resultant neurological or psychological impairments, under the criteria for the affected body system.
7. Primary peritoneal carcinoma. We use the criteria in 13.23E to evaluate primary peritoneal carcinoma in women because this cancer is often indistinguishable from ovarian cancer and is generally treated the same way as ovarian cancer. We use the criteria in 13.15A to evaluate primary peritoneal carcinoma in men because many of these cases are similar to malignant mesothelioma.
8. Prostate cancer. We exclude “biochemical recurrence” in 13.24A, which is defined as an increase in the serum prostate-specific antigen (PSA) level following the completion of the hormonal intervention therapy. We need corroborating evidence to document recurrence, such as radiological studies or findings on physical examination.
9. Melanoma. We evaluate malignant melanoma that affects the skin (cutaneous melanoma), eye (ocular melanoma), or mucosal membranes (mucosal melanoma) under 13.29. We evaluate melanoma that is not malignant that affects the skin (benign melanocytic tumor) under the listings in 8.00 or other affected body systems.
L. How do we evaluate cancer treated by bone marrow or stem cell transplantation, including transplantation using stem cells from umbilical cord blood? Bone marrow or stem cell transplantation is performed for a variety of cancers. We require the transplantation to occur before we evaluate it under these listings. We do not need to restrict our determination of the onset of disability to the date of the transplantation (13.05, 13.06, or 13.07) or the date of first treatment under the treatment plan that includes transplantation (13.28). We may be able to establish an earlier onset date of disability due to your transplantation if the evidence in your case record supports such a finding.
1. Acute leukemia (including T-cell lymphoblastic lymphoma) or accelerated or blast phase of CML. If you undergo bone marrow or stem cell transplantation for any of these disorders, we will consider you to be disabled until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of transplantation, whichever is later.
2. Lymphoma, multiple myeloma, or chronic phase of CML. If you undergo bone marrow or stem cell transplantation for any of these disorders, we will consider you to be disabled until at least 12 months from the date of transplantation.
3. Other cancers. We will evaluate any other cancer treated with bone marrow or stem cell transplantation under 13.28, regardless of whether there is another listing that addresses that impairment. The length of time we will consider you to be disabled depends on whether you undergo allogeneic or autologous transplantation.
a. Allogeneic bone marrow or stem cell transplantation. If you undergo allogeneic transplantation (transplantation from an unrelated donor or a related donor other than an identical twin), we will consider you to be disabled until at least 12 months from the date of transplantation.
b. Autologous bone marrow or stem cell transplantation. If you undergo autologous transplantation (transplantation of your own cells or cells from your identical twin (syngeneic transplantation)), we will consider you to be disabled until at least 12 months from the date of the first treatment under the treatment plan that includes transplantation. The first treatment usually refers to the initial therapy given to prepare you for transplantation.
4. Evaluating disability after the appropriate time period has elapsed. We consider any residual impairment(s), such as complications arising from:
a. Graft-versus-host (GVH) disease.
b. Immunosuppressant therapy, such as frequent infections.
c. Significant deterioration of other organ systems.
13.01 Category of Impairments, Malignant Neoplastic Diseases13.02 Soft tissue cancers of the head and neck (except salivary glands - 13.08 - and thyroid gland - 13.09).
A. Inoperable or unresectable.
ORB. Persistent or recurrent disease following initial anticancer therapy, except persistence or recurrence in the true vocal cord.
ORC. With metastases beyond the regional lymph nodes.
ORD. Small-cell (oat cell) carcinoma.
ORE. Soft tissue cancers originating in the head and neck treated with multimodal anticancer therapy (see 13.00E3c). Consider under a disability until at least 18 months from the date of diagnosis. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.03 Skin.
A. Sarcoma or carcinoma with metastases to or beyond the regional lymph nodes.
ORB. Carcinoma invading deep extradermal structures (for example, skeletal muscle, cartilage, or bone).
13.04 Soft tissue sarcoma.
A. With regional or distant metastases.
ORB. Persistent or recurrent following initial anticancer therapy.
13.05 Lymphoma (including mycosis fungoides, but excluding T-cell lymphoblastic lymphoma - 13.06). (See 13.00K1 and 13.00K2c.)
A. Non-Hodgkin's lymphoma, as described in 1 or 2:
1. Aggressive lymphoma (including diffuse large B-cell lymphoma) persistent or recurrent following initial anticancer therapy.
2. Indolent lymphoma (including mycosis fungoides and follicular small cleaved cell) requiring initiation of more than one (single mode or multimodal) anticancer treatment regimen within a period of 12 consecutive months. Consider under a disability from at least the date of initiation of the treatment regimen that failed within 12 months.
ORB. Hodgkin lymphoma with failure to achieve clinically complete remission, or recurrent lymphoma within 12 months of completing initial anticancer therapy.
ORC. With bone marrow or stem cell transplantation. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
ORD. Mantle cell lymphoma.
13.06 Leukemia. (See 13.00K2.)
A. Acute leukemia (including T-cell lymphoblastic lymphoma). Consider under a disability until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of bone marrow or stem cell transplantation, whichever is later. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
ORB. Chronic myelogenous leukemia, as described in 1 or 2:
1. Accelerated or blast phase (see 13.00K2b). Consider under a disability until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of bone marrow or stem cell transplantation, whichever is later. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
2. Chronic phase, as described in a or b:
a. Consider under a disability until at least 12 months from the date of bone marrow or stem cell transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
b. Progressive disease following initial anticancer therapy.
13.07 Multiple myeloma (confirmed by appropriate serum or urine protein electrophoresis and bone marrow findings).
A. Failure to respond or progressive disease following initial anticancer therapy.
ORB. With bone marrow or stem cell transplantation. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.08 Salivary glands - carcinoma or sarcoma with metastases beyond the regional lymph nodes.
13.09 Thyroid gland.
A. Anaplastic (undifferentiated) carcinoma.
ORB. Carcinoma with metastases beyond the regional lymph nodes progressive despite radioactive iodine therapy.
ORC. Medullary carcinoma with metastases beyond the regional lymph nodes.
13.10 Breast (except sarcoma - 13.04). (See 13.00K4.)
A. Locally advanced cancer (inflammatory carcinoma, cancer of any size with direct extension to the chest wall or skin, or cancer of any size with metastases to the ipsilateral internal mammary nodes).
ORB. Carcinoma with metastases to the supraclavicular or infraclavicular nodes, to 10 or more axillary nodes, or with distant metastases.
ORC. Recurrent carcinoma, except local recurrence that remits with anticancer therapy.
ORD. Small-cell (oat cell) carcinoma.
ORE. With secondary lymphedema that is caused by anticancer therapy and treated by surgery to salvage or restore the functioning of an upper extremity. (See 13.00K4b.) Consider under a disability until at least 12 months from the date of the surgery that treated the secondary lymphedema. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.11 Skeletal system - sarcoma.
A. Inoperable or unresectable.
ORB. Recurrent cancer (except local recurrence) after initial anticancer therapy.
ORC. With distant metastases.
ORD. All other cancers originating in bone with multimodal anticancer therapy (see 13.00E3c). Consider under a disability for 12 months from the date of diagnosis. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.12 Maxilla, orbit, or temporal fossa.
A. Sarcoma or carcinoma of any type with regional or distant metastases.
ORB. Carcinoma of the antrum with extension into the orbit or ethmoid or sphenoid sinus.
ORC. Cancer with extension to the orbit, meninges, sinuses, or base of the skull.
13.13 Nervous system. (See 13.00K6.)
A. Primary central nervous system (CNS; that is, brain and spinal cord) cancers, as described in 1, 2, or 3:
1. Glioblastoma multiforme, ependymoblastoma, and diffuse intrinsic brain stem gliomas (see 13.00K6a).
2. Any Grade III or Grade IV CNS cancer (see 13.00K6b), including astrocytomas, sarcomas, and medulloblastoma and other primitive neuroectodermal tumors (PNETs).
3. Any primary CNS cancer, as described in a or b:
a. Metastatic.
b. Progressive or recurrent following initial anticancer therapy.
ORB. Primary peripheral nerve or spinal root cancers, as described in 1 or 2:
1. Metastatic.
2. Progressive or recurrent following initial anticancer therapy.
13.14 Lungs.
A. Non-small-cell carcinoma - inoperable, unresectable, recurrent, or metastatic disease to or beyond the hilar nodes.
ORB. Small-cell (oat cell) carcinoma.
ORC. Carcinoma of the superior sulcus (including Pancoast tumors) with multimodal anticancer therapy (see 13.00E3c). Consider under a disability until at least 18 months from the date of diagnosis. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.15 Pleura or mediastinum.
A. Malignant mesothelioma of pleura.
ORB. Tumors of the mediastinum, as described in 1 or 2:
1. With metastases to or beyond the regional lymph nodes.
2. Persistent or recurrent following initial anticancer therapy.
ORC. Small-cell (oat cell) carcinoma.
13.16 Esophagus or stomach.
A. Carcinoma or sarcoma of the esophagus.
OR
B. Carcinoma or sarcoma of the stomach, as described in 1 or 2:
1. Inoperable, unresectable, extending to surrounding structures, or recurrent.
2. With metastases to or beyond the regional lymph nodes.
ORC. Small-cell (oat cell) carcinoma.
13.17 Small intestine - carcinoma, sarcoma, or carcinoid.
A. Inoperable, unresectable, or recurrent.
ORB. With metastases beyond the regional lymph nodes.
ORC. Small-cell (oat cell) carcinoma.
13.18 Large intestine (from ileocecal valve to and including anal canal).
A. Adenocarcinoma that is inoperable, unresectable, or recurrent.
ORB. Squamous cell carcinoma of the anus, recurrent after surgery.
ORC. With metastases beyond the regional lymph nodes.
ORD. Small-cell (oat cell) carcinoma.
13.19 Liver or gallbladder - cancer of the liver, gallbladder, or bile ducts.
13.20 Pancreas.
A. Carcinoma (except islet cell carcinoma).
ORB. Islet cell carcinoma that is physiologically active and is either inoperable or unresectable.
13.21 Kidneys, adrenal glands, or ureters - carcinoma.
A. Inoperable, unresectable, or recurrent.
ORB. With metastases to or beyond the regional lymph nodes.
13.22 Urinary bladder - carcinoma.
A. With infiltration beyond the bladder wall.
ORB. Recurrent after total cystectomy.
ORC. Inoperable or unresectable.
ORD. With metastases to or beyond the regional lymph nodes.
ORE. Small-cell (oat cell) carcinoma.
13.23 Cancers of the female genital tract - carcinoma or sarcoma (including primary peritoneal carcinoma).
A. Uterus (corpus), as described in 1, 2, or 3:
1. Invading adjoining organs.
2. With metastases to or beyond the regional lymph nodes.
3. Persistent or recurrent following initial anticancer therapy.
ORB. Uterine cervix, as described in 1, 2, or 3:
1. Extending to the pelvic wall, lower portion of the vagina, or adjacent or distant organs.
2. Persistent or recurrent following initial anticancer therapy.
3. With metastases to distant (for example, para-aortic or supraclavicular) lymph nodes.
ORC. Vulva or vagina, as described in 1, 2, or 3:
1. Invading adjoining organs.
2. With metastases to or beyond the regional lymph nodes.
3. Persistent or recurrent following initial anticancer therapy.
ORD. Fallopian tubes, as described in 1 or 2:
1. Extending to the serosa or beyond.
2. Persistent or recurrent following initial anticancer therapy.
E. Ovaries, as described in 1 or 2:
1. All cancers except germ-cell cancers, with at least one of the following:
a. Extension beyond the pelvis; for example, implants on, or direct extension to, peritoneal, omental, or bowel surfaces.
b. Metastases to or beyond the regional lymph nodes.
c. Recurrent following initial anticancer therapy.
2. Germ-cell cancers - progressive or recurrent following initial anticancer therapy.
ORF. Small-cell (oat cell) carcinoma.
13.24 Prostate gland - carcinoma.
A. Progressive or recurrent (not including biochemical recurrence) despite initial hormonal intervention. (See 13.00K8.)
ORB. With visceral metastases (metastases to internal organs).
ORC. Small cell (oat cell) carcinoma.
13.25 Testicles - cancer with metastatic disease progressive or recurrent following initial chemotherapy.
13.26 Penis - carcinoma with metastases to or beyond the regional lymph nodes.
13.27 Primary site unknown after appropriate search for primary - metastatic carcinoma or sarcoma, except for squamous cell carcinoma confined to the neck nodes.
13.28 Cancer treated by bone marrow or stem cell transplantation. (See 13.00L.)
A. Allogeneic transplantation. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
ORB. Autologous transplantation. Consider under a disability until at least 12 months from the date of the first treatment under the treatment plan that includes transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
13.29 Malignant melanoma (including skin, ocular, or mucosal melanomas), as described in either A, B, or C:
A. Recurrent (except an additional primary melanoma at a different site, which is not considered to be recurrent disease) following either 1 or 2:
1. Wide excision (skin melanoma).
2. Enucleation of the eye (ocular melanoma).
ORB. With metastases as described in 1, 2, or 3:
1. Metastases to one or more clinically apparent nodes; that is, nodes that are detected by imaging studies (excluding lymphoscintigraphy) or by clinical evaluation (palpable).
2. If the nodes are not clinically apparent, with metastases to four or more nodes.
3. Metastases to adjacent skin (satellite lesions) or distant sites (for example, liver, lung, or brain).
ORC. Mucosal melanoma.
14.00 Immune System DisordersA. What disorders do we evaluate under the immune system disorders listings?
1. We evaluate immune system disorders that cause dysfunction in one or more components of your immune system.
a. The dysfunction may be due to problems in antibody production, impaired cell-mediated immunity, a combined type of antibody/cellular deficiency, impaired phagocytosis, or complement deficiency.
b. Immune system disorders may result in recurrent and unusual infections, or inflammation and dysfunction of the body's own tissues. Immune system disorders can cause a deficit in a single organ or body system that results in extreme (that is, very serious) loss of function. They can also cause lesser degrees of limitations in two or more organs or body systems, and when associated with symptoms or signs, such as severe fatigue, fever, malaise, diffuse musculoskeletal pain, or involuntary weight loss, can also result in extreme limitation.
c. We organize the discussions of immune system disorders in three categories: Autoimmune disorders; Immune deficiency disorders, excluding human immunodeficiency virus (HIV) infection; and HIV infection.
2. Autoimmune disorders (14.00D). Autoimmune disorders are caused by dysfunctional immune responses directed against the body's own tissues, resulting in chronic, multisystem impairments that differ in clinical manifestations, course, and outcome. They are sometimes referred to as rheumatic diseases, connective tissue disorders, or collagen vascular disorders. Some of the features of autoimmune disorders in adults differ from the features of the same disorders in children.
3. Immune deficiency disorders, excluding HIV infection (14.00E). Immune deficiency disorders are characterized by recurrent or unusual infections that respond poorly to treatment, and are often associated with complications affecting other parts of the body. Immune deficiency disorders are classified as either primary (congenital) or acquired. Individuals with immune deficiency disorders also have an increased risk of malignancies and of having autoimmune disorders.
4. Human immunodeficiency virus (HIV) infection (14.00F). HIV infection may be characterized by increased susceptibility to common infections as well as opportunistic infections, cancers, or other conditions listed in 14.11.
B. What information do we need to show that you have an immune system disorder? Generally, we need your medical history, a report(s) of a physical examination, a report(s) of laboratory findings, and in some instances, appropriate medically acceptable imaging or tissue biopsy reports to show that you have an immune system disorder. Therefore, we will make every reasonable effort to obtain your medical history, medical findings, and results of laboratory tests. We explain the information we need in more detail in the sections below.
C. Definitions1. Appropriate medically acceptable imaging includes, but is not limited to, angiography, x-ray imaging, computerized axial tomography (CAT scan) or magnetic resonance imaging (MRI), with or without contrast material, myelography, and radionuclear bone scans. “Appropriate” means that the technique used is the proper one to support the evaluation and diagnosis of the impairment.
2. Constitutional symptoms or signs, as used in these listings, means severe fatigue, fever, malaise, or involuntary weight loss. Severe fatigue means a frequent sense of exhaustion that results in significantly reduced physical activity or mental function. Malaise means frequent feelings of illness, bodily discomfort, or lack of well-being that result in significantly reduced physical activity or mental function.
3. Disseminated means that a condition is spread over a considerable area. The type and extent of the spread will depend on your specific disease.
4. Dysfunction means that one or more of the body regulatory mechanisms are impaired, causing either an excess or deficiency of immunocompetent cells or their products.
5. Extra-articular means “other than the joints”; for example, an organ(s) such as the heart, lungs, kidneys, or skin.
6. Inability to ambulate effectively has the same meaning as in 1.00B2b.
7. Inability to perform fine and gross movements effectively has the same meaning as in 1.00B2c.
8. Major peripheral joints has the same meaning as in 1.00F.
9. Persistent means that a sign(s) or symptom(s) has continued over time. The precise meaning will depend on the specific immune system disorder, the usual course of the disorder, and the other circumstances of your clinical course.
10. Recurrent means that a condition that previously responded adequately to an appropriate course of treatment returns after a period of remission or regression. The precise meaning, such as the extent of response or remission and the time periods involved, will depend on the specific disease or condition you have, the body system affected, the usual course of the disorder and its treatment, and the other facts of your particular case.
11. Resistant to treatment means that a condition did not respond adequately to an appropriate course of treatment. Whether a response is adequate or a course of treatment is appropriate will depend on the specific disease or condition you have, the body system affected, the usual course of the disorder and its treatment, and the other facts of your particular case.
12. Severe means medical severity as used by the medical community. The term does not have the same meaning as it does when we use it in connection with a finding at the second step of the sequential evaluation processes in §§ 404.1520, 416.920, and 416.924.
D. How do we document and evaluate the listed autoimmune disorders?1. Systemic lupus erythematosus (14.02).
a. General. Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that can affect any organ or body system. It is frequently, but not always, accompanied by constitutional symptoms or signs (severe fatigue, fever, malaise, involuntary weight loss). Major organ or body system involvement can include: Respiratory (pleuritis, pneumonitis), cardiovascular (endocarditis, myocarditis, pericarditis, vasculitis), renal (glomerulonephritis), hematologic (anemia, leukopenia, thrombocytopenia), skin (photosensitivity), neurologic (seizures), mental (anxiety, fluctuating cognition (“lupus fog”), mood disorders, organic brain syndrome, psychosis), or immune system disorders (inflammatory arthritis). Immunologically, there is an array of circulating serum auto-antibodies and pro- and anti-coagulant proteins that may occur in a highly variable pattern.
b. Documentation of SLE. Generally, but not always, the medical evidence will show that your SLE satisfies the criteria in the current “Criteria for the Classification of Systemic Lupus Erythematosus” by the American College of Rheumatology found in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
2. Systemic vasculitis (14.03).
a. General.
(i) Vasculitis is an inflammation of blood vessels. It may occur acutely in association with adverse drug reactions, certain chronic infections, and occasionally, malignancies. More often, it is chronic and the cause is unknown. Symptoms vary depending on which blood vessels are involved. Systemic vasculitis may also be associated with other autoimmune disorders; for example, SLE or dermatomyositis.
(ii) There are several clinical patterns, including but not limited to polyarteritis nodosa, Takayasu's arteritis (aortic arch arteritis), giant cell arteritis (temporal arteritis), and Wegener's granulomatosis.
b. Documentation of systemic vasculitis. Angiography or tissue biopsy confirms a diagnosis of systemic vasculitis when the disease is suspected clinically. When you have had angiography or tissue biopsy for systemic vasculitis, we will make every reasonable effort to obtain reports of the results of that procedure. However, we will not purchase angiography or tissue biopsy.
3. Systemic sclerosis (scleroderma) (14.04).
a. General. Systemic sclerosis (scleroderma) constitutes a spectrum of disease in which thickening of the skin is the clinical hallmark. Raynaud's phenomenon, often medically severe and progressive, is present frequently and may be the peripheral manifestation of a vasospastic abnormality in the heart, lungs, and kidneys. The CREST syndrome (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) is a variant that may slowly progress over years to the generalized process, systemic sclerosis.
b. Diffuse cutaneous systemic sclerosis. In diffuse cutaneous systemic sclerosis (also known as diffuse scleroderma), major organ or systemic involvement can include the gastrointestinal tract, lungs, heart, kidneys, and muscle in addition to skin or blood vessels. Although arthritis can occur, joint dysfunction results primarily from soft tissue/cutaneous thickening, fibrosis, and contractures.
c. Localized scleroderma (linear scleroderma and morphea).
(i) Localized scleroderma (linear scleroderma and morphea) is more common in children than in adults. However, this type of scleroderma can persist into adulthood. To assess the severity of the impairment, we need a description of the extent of involvement of linear scleroderma and the location of the lesions. For example, linear scleroderma involving the arm but not crossing any joints is not as functionally limiting as sclerodactyly (scleroderma localized to the fingers). Linear scleroderma of a lower extremity involving skin thickening and atrophy of underlying muscle or bone can result in contractures and leg length discrepancy. In such cases, we may evaluate your impairment under the musculoskeletal listings (1.00).
(ii) When there is isolated morphea of the face causing facial disfigurement from unilateral hypoplasia of the mandible, maxilla, zygoma, or orbit, adjudication may be more appropriate under the criteria in the affected body system, such as special senses and speech (2.00) or mental disorders (12.00).
(iii) Chronic variants of these syndromes include disseminated morphea, Shulman's disease (diffuse fasciitis with eosinophilia), and eosinophilia-myalgia syndrome (often associated with toxins such as toxic oil or contaminated tryptophan), all of which can impose medically severe musculoskeletal dysfunction and may also lead to restrictive pulmonary disease. We evaluate these variants of the disease under the criteria in the musculoskeletal listings (1.00) or respiratory system listings (3.00).
d. Documentation of systemic sclerosis (scleroderma). Documentation involves differentiating the clinical features of systemic sclerosis (scleroderma) from other autoimmune disorders. However, there may be an overlap.
4. Polymyositis and dermatomyositis (14.05).
a. General. Polymyositis and dermatomyositis are related disorders that are characterized by an inflammatory process in striated muscle, occurring alone or in association with other autoimmune disorders or malignancy. The most common manifestations are symmetric weakness, and less frequently, pain and tenderness of the proximal limb-girdle (shoulder or pelvic) musculature. There may also be involvement of the cervical, cricopharyngeal, esophageal, intercostal, and diaphragmatic muscles.
b. Documentation of polymyositis and dermatomyositis. Generally, but not always, polymyositis is associated with elevated serum muscle enzymes (creatine phosphokinase (CPK), aminotransferases, and aldolase), and characteristic abnormalities on electromyography and muscle biopsy. In dermatomyositis there are characteristic skin findings in addition to the findings of polymyositis. When you have had electromyography or muscle biopsy for polymyositis or dermatomyositis, we will make every reasonable effort to obtain reports of the results of that procedure. However, we will not purchase electromyography or muscle biopsy.
c. Additional information about how we evaluate polymyositis and dermatomyositis under the listings.
(i) Weakness of your pelvic girdle muscles that results in your inability to rise independently from a squatting or sitting position or to climb stairs may be an indication that you are unable to ambulate effectively. Weakness of your shoulder girdle muscles may result in your inability to perform lifting, carrying, and reaching overhead, and also may seriously affect your ability to perform activities requiring fine movements. We evaluate these limitations under 14.05A.
(ii) We use the malignant neoplastic diseases listings (13.00) to evaluate malignancies associated with polymyositis or dermatomyositis. We evaluate the involvement of other organs/body systems under the criteria for the listings in the affected body system.
5. Undifferentiated and mixed connective tissue disease (14.06).
a. General. This listing includes syndromes with clinical and immunologic features of several autoimmune disorders, but which do not satisfy the criteria for any of the specific disorders described. For example, you may have clinical features of SLE and systemic vasculitis, and the serologic (blood test) findings of rheumatoid arthritis.
b. Documentation of undifferentiated and mixed connective tissue disease. Undifferentiated connective tissue disease is diagnosed when clinical features and serologic (blood test) findings, such as rheumatoid factor or antinuclear antibody (consistent with an autoimmune disorder) are present but do not satisfy the criteria for a specific disease. Mixed connective tissue disease (MCTD) is diagnosed when clinical features and serologic findings of two or more autoimmune diseases overlap.
6. Inflammatory arthritis (14.09).
a. General. The spectrum of inflammatory arthritis includes a vast array of disorders that differ in cause, course, and outcome. Clinically, inflammation of major peripheral joints may be the dominant manifestation causing difficulties with ambulation or fine and gross movements; there may be joint pain, swelling, and tenderness. The arthritis may affect other joints, or cause less limitation in ambulation or the performance of fine and gross movements. However, in combination with extra-articular features, including constitutional symptoms or signs (severe fatigue, fever, malaise, involuntary weight loss), inflammatory arthritis may result in an extreme limitation.
b. Inflammatory arthritis involving the axial spine (spondyloarthropathy). In adults, inflammatory arthritis involving the axial spine may be associated with disorders such as:
(i) Reiter's syndrome;
(ii) Ankylosing spondylitis;
(iii) Psoriatic arthritis;
(iv) Whipple's disease;
(v) Behçet's disease; and
(vi) Inflammatory bowel disease.
c. Inflammatory arthritis involving the peripheral joints. In adults, inflammatory arthritis involving peripheral joints may be associated with disorders such as:
(i) Rheumatoid arthritis;
(ii) Sjögren's syndrome;
(iii) Psoriatic arthritis;
(iv) Crystal deposition disorders (gout and pseudogout);
(v) Lyme disease; and
(vi) Inflammatory bowel disease.
d. Documentation of inflammatory arthritis. Generally, but not always, the diagnosis of inflammatory arthritis is based on the clinical features and serologic findings described in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
e. How we evaluate inflammatory arthritis under the listings.
(i) Listing-level severity in 14.09A and 14.09C1 is shown by an impairment that results in an “extreme” (very serious) limitation. In 14.09A, the criterion is satisfied with persistent inflammation or deformity in one major peripheral weight-bearing joint resulting in the inability to ambulate effectively (as defined in 14.00C6) or one major peripheral joint in each upper extremity resulting in the inability to perform fine and gross movements effectively (as defined in 14.00C7). In 14.09C1, if you have the required ankylosis (fixation) of your cervical or dorsolumbar spine, we will find that you have an extreme limitation in your ability to see in front of you, above you, and to the side. Therefore, inability to ambulate effectively is implicit in 14.09C1, even though you might not require bilateral upper limb assistance.
(ii) Listing-level severity is shown in 14.09B, 14.09C2, and 14.09D by inflammatory arthritis that involves various combinations of complications of one or more major peripheral joints or other joints, such as inflammation or deformity, extra-articular features, repeated manifestations, and constitutional symptoms or signs. Extra-articular impairments may also meet listings in other body systems.
(iii) Extra-articular features of inflammatory arthritis may involve any body system; for example: Musculoskeletal (heel enthesopathy), ophthalmologic (iridocyclitis, keratoconjunctivitis sicca, uveitis), pulmonary (pleuritis, pulmonary fibrosis or nodules, restrictive lung disease), cardiovascular (aortic valve insufficiency, arrhythmias, coronary arteritis, myocarditis, pericarditis, Raynaud's phenomenon, systemic vasculitis), renal (amyloidosis of the kidney), hematologic (chronic anemia, thrombocytopenia), neurologic (peripheral neuropathy, radiculopathy, spinal cord or cauda equina compression with sensory and motor loss), mental (cognitive dysfunction, poor memory), and immune system (Felty's syndrome (hypersplenism with compromised immune competence)).
(iv) If both inflammation and chronic deformities are present, we evaluate your impairment under the criteria of any appropriate listing.
7. Sjögren's syndrome (14.10).
a. General.
(i) Sjögren's syndrome is an immune-mediated disorder of the exocrine glands. Involvement of the lacrimal and salivary glands is the hallmark feature, resulting in symptoms of dry eyes and dry mouth, and possible complications, such as corneal damage, blepharitis (eyelid inflammation), dysphagia (difficulty in swallowing), dental caries, and the inability to speak for extended periods of time. Involvement of the exocrine glands of the upper airways may result in persistent dry cough.
(ii) Many other organ systems may be involved, including musculoskeletal (arthritis, myositis), respiratory (interstitial fibrosis), gastrointestinal (dysmotility, dysphagia, involuntary weight loss), genitourinary (interstitial cystitis, renal tubular acidosis), skin (purpura, vasculitis), neurologic (central nervous system disorders, cranial and peripheral neuropathies), mental (cognitive dysfunction, poor memory), and neoplastic (lymphoma). Severe fatigue and malaise are frequently reported. Sjögren's syndrome may be associated with other autoimmune disorders (for example, rheumatoid arthritis or SLE); usually the clinical features of the associated disorder predominate.
b. Documentation of Sjögren's syndrome. If you have Sjögren's syndrome, the medical evidence will generally, but not always, show that your disease satisfies the criteria in the current “Criteria for the Classification of Sjögren's Syndrome” by the American College of Rheumatology found in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
E. How do we document and evaluate immune deficiency disorders, excluding HIV infection?1. General.
a. Immune deficiency disorders can be classified as:
(i) Primary (congenital); for example, X-linked agammaglobulinemia, thymic hypoplasia (DiGeorge syndrome), severe combined immunodeficiency (SCID), chronic granulomatous disease (CGD), C1 esterase inhibitor deficiency.
(ii) Acquired; for example, medication-related.
b. Primary immune deficiency disorders are seen mainly in children. However, recent advances in the treatment of these disorders have allowed many affected children to survive well into adulthood. Occasionally, these disorders are first diagnosed in adolescence or adulthood.
2. Documentation of immune deficiency disorders. The medical evidence must include documentation of the specific type of immune deficiency. Documentation may be by laboratory evidence or by other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice.
3. Immune deficiency disorders treated by stem cell transplantation.
a. Evaluation in the first 12 months. If you undergo stem cell transplantation for your immune deficiency disorder, we will consider you disabled until at least 12 months from the date of the transplant.
b. Evaluation after the 12-month period has elapsed. After the 12-month period has elapsed, we will consider any residuals of your immune deficiency disorder as well as any residual impairment(s) resulting from the treatment, such as complications arising from:
(i) Graft-versus-host (GVH) disease.
(ii) Immunosuppressant therapy, such as frequent infections.
(iii) Significant deterioration of other organ systems.
4. Medication-induced immune suppression. Medication effects can result in varying degrees of immune suppression, but most resolve when the medication is ceased. However, if you are prescribed medication for long-term immune suppression, such as after an organ transplant, we will evaluate:
a. The frequency and severity of infections.
b. Residuals from the organ transplant itself, after the 12-month period has elapsed.
c. Significant deterioration of other organ systems.
F. How do we document and evaluate HIV infection? Any individual with HIV infection, including one with a diagnosis of acquired immune deficiency syndrome (AIDS), may be found disabled under 14.11 if his or her impairment meets the criteria in that listing or is medically equivalent to the criteria in that listing.
1. Documentation of HIV infection.
a. Definitive documentation of HIV infection. We may document a diagnosis of HIV infection by positive findings on one or more of the following definitive laboratory tests:
(i) HIV antibody screening test (for example, enzyme immunoassay, or EIA), confirmed by a supplemental HIV antibody test such as the Western blot (immunoblot), an immunofluorescence assay, or an HIV-1/HIV-2 antibody differentiation immunoassay.
(ii) HIV nucleic acid (DNA or RNA) detection test (for example, polymerase chain reaction, or PCR).
(iii) HIV p24 antigen (p24Ag) test.
(iv) Isolation of HIV in viral culture.
(v) Other tests that are highly specific for detection of HIV and that are consistent with the prevailing state of medical knowledge.
b. We will make every reasonable effort to obtain the results of your laboratory testing. Pursuant to §§ 404.1519f and 416.919f of this chapter, we will purchase examinations or tests necessary to make a determination in your claim if no other acceptable documentation exists.
c. Other acceptable documentation of HIV infection. We may also document HIV infection without definitive laboratory evidence.
(i) We will accept a persuasive report from a physician that a positive diagnosis of your HIV infection was confirmed by an appropriate laboratory test(s), such as those described in 14.00F1a. To be persuasive, this report must state that you had the appropriate definitive laboratory test(s) for diagnosing your HIV infection and provide the results. The report must also be consistent with the remaining evidence of record.
(ii) We may also document HIV infection by the medical history, clinical and laboratory findings, and diagnosis(es) indicated in the medical evidence, provided that such documentation is consistent with the prevailing state of medical knowledge and clinical practice and is consistent with the other evidence in your case record. For example, we will accept a diagnosis of HIV infection without definitive laboratory evidence of the HIV infection if you have an opportunistic disease that is predictive of a defect in cell-mediated immunity (for example, toxoplasmosis of the brain or Pneumocystis pneumonia (PCP)), and there is no other known cause of diminished resistance to that disease (for example, long-term steroid treatment or lymphoma). In such cases, we will make every reasonable effort to obtain full details of the history, medical findings, and results of testing.
2. Documentation of the manifestations of HIV infection.
a. Definitive documentation of manifestations of HIV infection. We may document manifestations of HIV infection by positive findings on definitive laboratory tests, such as culture, microscopic examination of biopsied tissue or other material (for example, bronchial washings), serologic tests, or on other generally acceptable definitive tests consistent with the prevailing state of medical knowledge and clinical practice.
b. We will make every reasonable effort to obtain the results of your laboratory testing. Pursuant to §§ 404.1519f and 416.919f of this chapter, we will purchase examinations or tests necessary to make a determination of your claim if no other acceptable documentation exists.
c. Other acceptable documentation of manifestations of HIV infection. We may also document manifestations of HIV infection without definitive laboratory evidence.
(i) We will accept a persuasive report from a physician that a positive diagnosis of your manifestation of HIV infection was confirmed by an appropriate laboratory test(s). To be persuasive, this report must state that you had the appropriate definitive laboratory test(s) for diagnosing your manifestation of HIV infection and provide the results. The report must also be consistent with the remaining evidence of record.
(ii) We may also document manifestations of HIV infection without the definitive laboratory evidence described in 14.00F2a, provided that such documentation is consistent with the prevailing state of medical knowledge and clinical practice and is consistent with the other evidence in your case record. For example, many conditions are now commonly diagnosed based on some or all of the following: Medical history, clinical manifestations, laboratory findings (including appropriate medically acceptable imaging), and treatment responses. In such cases, we will make every reasonable effort to obtain full details of the history, medical findings, and results of testing.
3. Disorders associated with HIV infection (14.11A-E).
a. Multicentric Castleman disease (MCD, 14.11A) affects multiple groups of lymph nodes and organs containing lymphoid tissue. This widespread involvement distinguishes MCD from localized (or unicentric) Castleman disease, which affects only a single set of lymph nodes. While not a cancer, MCD is known as a lymphoproliferative disorder. Its clinical presentation and progression is similar to that of lymphoma, and its treatment may include radiation or chemotherapy. We require characteristic findings on microscopic examination of the biopsied lymph nodes or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis. Localized (or unicentric) Castleman disease does not meet or medically equal the criterion in 14.11A, but we may evaluate it under the criteria in 14.11H or 14.11I.
b. Primary central nervous system lymphoma (PCNSL, 14.11B) originates in the brain, spinal cord, meninges, or eye. Imaging tests (for example, MRI) of the brain, while not diagnostic, may show a single lesion or multiple lesions in the white matter of the brain. We require characteristic findings on microscopic examination of the cerebral spinal fluid or of the biopsied brain tissue, or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
c. Primary effusion lymphoma (PEL, 14.11C) is also known as body cavity lymphoma. We require characteristic findings on microscopic examination of the effusion fluid or of the biopsied tissue from the affected internal organ, or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
d. Progressive multifocal leukoencephalopathy (PML, 14.11D) is a progressive neurological degenerative syndrome caused by the John Cunningham (JC) virus in immunosuppressed individuals. Clinical findings of PML include clumsiness, progressive weakness, and visual and speech changes. Personality and cognitive changes may also occur. We require appropriate clinical findings, characteristic white matter lesions on MRI, and a positive PCR test for the JC virus in the cerebrospinal fluid to establish the diagnosis. We also accept a positive brain biopsy for JC virus or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
e. Pulmonary Kaposi sarcoma (Kaposi sarcoma in the lung, 14.11E) is the most serious form of Kaposi sarcoma (KS). Other internal KS tumors (for example, tumors of the gastrointestinal tract) have a more variable prognosis. We require characteristic findings on microscopic examination of the induced sputum, bronchoalveolar lavage washings, or of the biopsied transbronchial tissue, or by other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
4. CD4 measurement (14.11F). To evaluate your HIV infection under 14.11F, we require one measurement of your absolute CD4 count (also known as CD4 count or CD4+ T-helper lymphocyte count). This measurement must occur within the period we are considering in connection with your application or continuing disability review. If you have more than one measurement of your absolute CD4 count within this period, we will use your lowest absolute CD4 count.
5. Measurement of CD4 and either body mass index or hemoglobin (14.11G). To evaluate your HIV infection under 14.11G, we require one measurement of your absolute CD4 count or your CD4 percentage, and either a measurement of your body mass index (BMI) or your hemoglobin. These measurements must occur within the period we are considering in connection with your application or continuing disability review. If you have more than one measurement of your CD4 (absolute count or percentage), BMI, or hemoglobin within this period, we will use the lowest of your CD4 (absolute count or percentage), BMI, or hemoglobin. The date of your lowest CD4 (absolute count or percentage) measurement may be different from the date of your lowest BMI or hemoglobin measurement. We calculate your BMI using the formulas in 5.00G2.
6. Complications of HIV infection requiring hospitalization (14.11H).
a. Complications of HIV infection may include infections (common or opportunistic), cancers, and other conditions. Examples of complications that may result in hospitalization include: Depression; diarrhea; immune reconstitution inflammatory syndrome; malnutrition; and PCP and other severe infections.
b. Under 14.11H, we require three hospitalizations within a 12-month period that are at least 30 days apart and that result from a complication(s) of HIV infection. The hospitalizations may be for the same complication or different complications of HIV infection and are not limited to the examples of complications that may result in hospitalization listed in 14.00F6a. All three hospitalizations must occur within the period we are considering in connection with your application or continuing disability review. Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
c. We will use the rules on medical equivalence in §§ 404.1526 and 416.926 of this chapter to evaluate your HIV infection if you have fewer, but longer, hospitalizations, or more frequent, but shorter, hospitalizations, or if you receive nursing, rehabilitation, or other care in alternative settings.
7. HIV infection manifestations specific to women.
a. General. Most women with severe immunosuppression secondary to HIV infection exhibit the typical opportunistic infections and other conditions, such as PCP, Candida esophagitis, wasting syndrome, cryptococcosis, and toxoplasmosis. However, HIV infection may have different manifestations in women than in men. Adjudicators must carefully scrutinize the medical evidence and be alert to the variety of medical conditions specific to, or common in, women with HIV infection that may affect their ability to function in the workplace.
b. Additional considerations for evaluating HIV infection in women. Many of these manifestations (for example, vulvovaginal candidiasis or pelvic inflammatory disease) occur in women with or without HIV infection, but can be more severe or resistant to treatment, or occur more frequently in a woman whose immune system is suppressed. Therefore, when evaluating the claim of a woman with HIV infection, it is important to consider gynecologic and other problems specific to women, including any associated symptoms (for example, pelvic pain), in assessing the severity of the impairment and resulting functional limitations. We may evaluate manifestations of HIV infection in women under 14.11H-I, or under the criteria for the appropriate body system (for example, cervical cancer under 13.23).
8. HIV-associated dementia (HAD). HAD is an advanced neurocognitive disorder, characterized by a significant decline in cognitive functioning. We evaluate HAD under 14.11I. Other names associated with neurocognitive disorders due to HIV infection include: AIDS dementia complex, HIV dementia, HIV encephalopathy, and major neurocognitive disorder due to HIV infection.
G. How do we consider the effects of treatment in evaluating your autoimmune disorder, immune deficiency disorder, or HIV infection?1. General. If your impairment does not otherwise meet the requirements of a listing, we will consider your medical treatment in terms of its effectiveness in improving the signs, symptoms, and laboratory abnormalities of your specific immune system disorder or its manifestations, and in terms of any side effects that limit your functioning. We will make every reasonable effort to obtain a specific description of the treatment you receive (including surgery) for your immune system disorder. We consider:
a. The effects of medications you take.
b. Adverse side effects (acute and chronic).
c. The intrusiveness and complexity of your treatment (for example, the dosing schedule, need for injections).
d. The effect of treatment on your mental functioning (for example, cognitive changes, mood disturbance).
e. Variability of your response to treatment (see 14.00G2).
f. The interactive and cumulative effects of your treatments. For example, many individuals with immune system disorders receive treatment both for their immune system disorders and for the manifestations of the disorders or co-occurring impairments, such as treatment for HIV infection and hepatitis C. The interactive and cumulative effects of these treatments may be greater than the effects of each treatment considered separately.
g. The duration of your treatment.
h. Any other aspects of treatment that may interfere with your ability to function.
2. Variability of your response to treatment. Your response to treatment and the adverse or beneficial consequences of your treatment may vary widely. The effects of your treatment may be temporary or long term. For example, some individuals may show an initial positive response to a drug or combination of drugs followed by a decrease in effectiveness. When we evaluate your response to treatment and how your treatment may affect you, we consider such factors as disease activity before treatment, requirements for changes in therapeutic regimens, the time required for therapeutic effectiveness of a particular drug or drugs, the limited number of drug combinations that may be available for your impairment(s), and the time-limited efficacy of some drugs. For example, an individual with HIV infection or another immune deficiency disorder who develops pneumonia or tuberculosis may not respond to the same antibiotic regimen used in treating individuals without HIV infection or another immune deficiency disorder, or may not respond to an antibiotic that he or she responded to before. Therefore, we must consider the effects of your treatment on an individual basis, including the effects of your treatment on your ability to function.
3. How we evaluate the effects of treatment for autoimmune disorders on your ability to function. Some medications may have acute or long-term side effects. When we consider the effects of corticosteroids or other treatments for autoimmune disorders on your ability to function, we consider the factors in 14.00G1 and 14.00G2. Long-term corticosteroid treatment can cause ischemic necrosis of bone, posterior subcapsular cataract, weight gain, glucose intolerance, increased susceptibility to infection, and osteoporosis that may result in a loss of function. In addition, medications used in the treatment of autoimmune disorders may also have effects on mental functioning, including cognition (for example, memory), concentration, and mood.
4. How we evaluate the effects of treatment for immune deficiency disorders, excluding HIV infection, on your ability to function. When we consider the effects of your treatment for your immune deficiency disorder on your ability to function, we consider the factors in 14.00G1 and 14.00G2. A frequent need for treatment such as intravenous immunoglobulin and gamma interferon therapy can be intrusive and interfere with your ability to work. We will also consider whether you have chronic side effects from these or other medications, including severe fatigue, fever, headaches, high blood pressure, joint swelling, muscle aches, nausea, shortness of breath, or limitations in mental function including cognition (for example, memory), concentration, and mood.
5. How we evaluate the effects of treatment for HIV infection on your ability to function.
a. General. When we consider the effects of antiretroviral drugs (including the effects of highly active antiretroviral therapy (HAART)) and the effects of treatments for the manifestations of HIV infection on your ability to function, we consider the factors in 14.00G1 and 14.00G2. Side effects of antiretroviral drugs include, but are not limited to: Bone marrow suppression, pancreatitis, gastrointestinal intolerance (nausea, vomiting, diarrhea), neuropathy, rash, hepatotoxicity, lipodystrophy (fat redistribution, such as “buffalo hump”), glucose intolerance, and lactic acidosis. In addition, medications used in the treatment of HIV infection may also have effects on mental functioning, including cognition (for example, memory), concentration, and mood, and may result in malaise, severe fatigue, joint and muscle pain, and insomnia. The symptoms of HIV infection and the side effects of medication may be indistinguishable from each other. We will consider all of your functional limitations, whether they result from your symptoms or signs of HIV infection or the side effects of your treatment.
b. Structured treatment interruptions. A structured treatment interruption (STI, also called a “drug holiday”) is a treatment practice during which your treating source advises you to stop taking your medications temporarily. An STI in itself does not imply that your medical condition has improved; nor does it imply that you are noncompliant with your treatment because you are following your treating source's advice. Therefore, if you have stopped taking medication because your treating source prescribed or recommended an STI, we will not find that you are failing to follow treatment or draw inferences about the severity of your impairment on this fact alone. We will consider why your treating source has prescribed or recommended an STI and all the other information in your case record when we determine the severity of your impairment.
6. When there is no record of ongoing treatment. If you have not received ongoing treatment or have not had an ongoing relationship with the medical community despite the existence of a severe impairment(s), we will evaluate the medical severity and duration of your immune system disorder on the basis of the current objective medical evidence and other evidence in your case record, taking into consideration your medical history, symptoms, clinical and laboratory findings, and medical source opinions. If you have just begun treatment and we cannot determine whether you are disabled based on the evidence we have, we may need to wait to determine the effect of the treatment on your ability to function. The amount of time we need to wait will depend on the facts of your case. If you have not received treatment, you may not be able to show an impairment that meets the criteria of one of the immune system disorders listings, but your immune system disorder may medically equal a listing or be disabling based on a consideration of your residual functional capacity, age, education, and work experience.
H. How do we consider your symptoms, including your pain, severe fatigue, and malaise?Your symptoms, including pain, severe fatigue, and malaise, may be important factors in our determination whether your immune system disorder(s) meets or medically equals a listing or in our determination whether you are otherwise able to work. In order for us to consider your symptoms, you must have medical signs or laboratory findings showing the existence of a medically determinable impairment(s) that could reasonably be expected to produce the symptoms. If you have such an impairment(s), we will evaluate the intensity, persistence, and functional effects of your symptoms using the rules throughout 14.00 and in our other regulations. See §§ 404.1521, 404.1529, 416.921, and 416.929. Additionally, when we assess the credibility of your complaints about your symptoms and their functional effects, we will not draw any inferences from the fact that you do not receive treatment or that you are not following treatment without considering all of the relevant evidence in your case record, including any explanations you provide that may explain why you are not receiving or following treatment.
I. How do we use the functional criteria in these listings?
1. The following listings in this body system include standards for evaluating the functional limitations resulting from immune system disorders: 14.02B, for systemic lupus erythematosus; 14.03B, for systemic vasculitis; 14.04D, for systemic sclerosis (scleroderma); 14.05E, for polymyositis and dermatomyositis; 14.06B, for undifferentiated and mixed connective tissue disease; 14.07C, for immune deficiency disorders, excluding HIV infection; 14.09D, for inflammatory arthritis; 14.10B, for Sjögren's syndrome; and 14.11I, for HIV infection.
2. When we use one of the listings cited in 14.00I1, we will consider all relevant information in your case record to determine the full impact of your immune system disorder on your ability to function on a sustained basis. Important factors we will consider when we evaluate your functioning under these listings include, but are not limited to: Your symptoms, the frequency and duration of manifestations of your immune system disorder, periods of exacerbation and remission, and the functional impact of your treatment, including the side effects of your medication.
3. As used in these listings, “repeated” means that the manifestations occur on an average of three times a year, or once every 4 months, each lasting 2 weeks or more; or the manifestations do not last for 2 weeks but occur substantially more frequently than three times in a year or once every 4 months; or they occur less frequently than an average of three times a year or once every 4 months but last substantially longer than 2 weeks. Your impairment will satisfy this criterion regardless of whether you have the same kind of manifestation repeatedly, all different manifestations, or any other combination of manifestations; for example, two of the same kind of manifestation and a different one. You must have the required number of manifestations with the frequency and duration required in this section. Also, the manifestations must occur within the period covered by your claim.
4. To satisfy the functional criterion in a listing, your immune system disorder must result in a “marked” level of limitation in one of three general areas of functioning: Activities of daily living, social functioning, or difficulties in completing tasks due to deficiencies in concentration, persistence, or pace. Functional limitation may result from the impact of the disease process itself on your mental functioning, physical functioning, or both your mental and physical functioning. This could result from persistent or intermittent symptoms, such as depression, severe fatigue, or pain, resulting in a limitation of your ability to do a task, to concentrate, to persevere at a task, or to perform the task at an acceptable rate of speed. You may also have limitations because of your treatment and its side effects (see 14.00G).
5. Marked limitation means that the signs and symptoms of your immune system disorder interfere seriously with your ability to function. Although we do not require the use of such a scale, “marked” would be the fourth point on a five-point scale consisting of no limitation, mild limitation, moderate limitation, marked limitation, and extreme limitation. You may have a marked limitation when several activities or functions are impaired, or even when only one is impaired. Also, you need not be totally precluded from performing an activity to have a marked limitation, as long as the degree of limitation seriously interferes with your ability to function independently, appropriately, and effectively. The term “marked” does not imply that you must be confined to bed, hospitalized, or in a nursing home.
6. Activities of daily living include, but are not limited to, such activities as doing household chores, grooming and hygiene, using a post office, taking public transportation, or paying bills. We will find that you have a “marked” limitation of activities of daily living if you have a serious limitation in your ability to maintain a household or take public transportation because of symptoms, such as pain, severe fatigue, anxiety, or difficulty concentrating, caused by your immune system disorder (including manifestations of the disorder) or its treatment, even if you are able to perform some self-care activities.
7. Social functioning includes the capacity to interact independently, appropriately, effectively, and on a sustained basis with others. It includes the ability to communicate effectively with others. We will find that you have a “marked” limitation in maintaining social functioning if you have a serious limitation in social interaction on a sustained basis because of symptoms, such as pain, severe fatigue, anxiety, or difficulty concentrating, or a pattern of exacerbation and remission, caused by your immune system disorder (including manifestations of the disorder) or its treatment, even if you are able to communicate with close friends or relatives.
8. Completing tasks in a timely manner involves the ability to sustain concentration, persistence, or pace to permit timely completion of tasks commonly found in work settings. We will find that you have a “marked” limitation in completing tasks if you have a serious limitation in your ability to sustain concentration or pace adequate to complete work-related tasks because of symptoms, such as pain, severe fatigue, anxiety, or difficulty concentrating, caused by your immune system disorder (including manifestations of the disorder) or its treatment, even if you are able to do some routine activities of daily living.
J. How do we evaluate your immune system disorder when it does not meet one of these listings?1. These listings are only examples of immune system disorders that we consider severe enough to prevent you from doing any gainful activity. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
2. Individuals with immune system disorders, including HIV infection, may manifest signs or symptoms of a mental impairment or of another physical impairment. For example, HIV infection may accelerate the onset of conditions such as diabetes or affect the course of or treatment options for diseases such as cardiovascular disease or hepatitis. We may evaluate these impairments under the affected body system.
3. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926.) If it does not, you may or may not have the residual functional capacity to engage in substantial gainful activity. Therefore, we proceed to the fourth, and if necessary, the fifth steps of the sequential evaluation process in §§ 404.1520 and 416.920. We use the rules in §§ 404.1594, 416.994, and 416.994a as appropriate, when we decide whether you continue to be disabled.
14.01 Category of Impairments, Immune System Disorders.
14.02 Systemic lupus erythematosus. As described in 14.00D1. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. Repeated manifestations of SLE, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.03 Systemic vasculitis. As described in 14.00D2. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. Repeated manifestations of systemic vasculitis, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.04 Systemic sclerosis (scleroderma). As described in 14.00D3. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. With one of the following:
1. Toe contractures or fixed deformity of one or both feet, resulting in the inability to ambulate effectively as defined in 14.00C6; or
2. Finger contractures or fixed deformity in both hands, resulting in the inability to perform fine and gross movements effectively as defined in 14.00C7; or
3. Atrophy with irreversible damage in one or both lower extremities, resulting in the inability to ambulate effectively as defined in 14.00C6; or
4. Atrophy with irreversible damage in both upper extremities, resulting in the inability to perform fine and gross movements effectively as defined in 14.00C7.
orC. Raynaud's phenomenon, characterized by:
1. Gangrene involving at least two extremities; or
2. Ischemia with ulcerations of toes or fingers, resulting in the inability to ambulate effectively or to perform fine and gross movements effectively as defined in 14.00C6 and 14.00C7;
orD. Repeated manifestations of systemic sclerosis (scleroderma), with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.05 Polymyositis and dermatomyositis. As described in 14.00D4. With:
A. Proximal limb-girdle (pelvic or shoulder) muscle weakness, resulting in inability to ambulate effectively or inability to perform fine and gross movements effectively as defined in 14.00C6 and 14.00C7.
orB. Impaired swallowing (dysphagia) with aspiration due to muscle weakness.
orC. Impaired respiration due to intercostal and diaphragmatic muscle weakness.
orD. Diffuse calcinosis with limitation of joint mobility or intestinal motility.
orE. Repeated manifestations of polymyositis or dermatomyositis, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.06 Undifferentiated and mixed connective tissue disease. As described in 14.00D5. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. Repeated manifestations of undifferentiated or mixed connective tissue disease, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.07 Immune deficiency disorders, excluding HIV infection. As described in 14.00E. With:
A. One or more of the following infections. The infection(s) must either be resistant to treatment or require hospitalization or intravenous treatment three or more times in a 12-month period.
1. Sepsis; or
2. Meningitis; or
3. Pneumonia; or
4. Septic arthritis; or
5. Endocarditis; or
6. Sinusitis documented by appropriate medically acceptable imaging.
orB. Stem cell transplantation as described under 14.00E3. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
orC. Repeated manifestations of an immune deficiency disorder, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social function.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.08 [Reserved]
14.09 Inflammatory arthritis. As described in 14.00D6. With:
A. Persistent inflammation or persistent deformity of:
1. One or more major peripheral weight-bearing joints resulting in the inability to ambulate effectively (as defined in 14.00C6); or
2. One or more major peripheral joints in each upper extremity resulting in the inability to perform fine and gross movements effectively (as defined in 14.00C7).
orB. Inflammation or deformity in one or more major peripheral joints with:
1. Involvement of two or more organs/body systems with one of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orC. Ankylosing spondylitis or other spondyloarthropathies, with:
1. Ankylosis (fixation) of the dorsolumbar or cervical spine as shown by appropriate medically acceptable imaging and measured on physical examination at 45° or more of flexion from the vertical position (zero degrees); or
2. Ankylosis (fixation) of the dorsolumbar or cervical spine as shown by appropriate medically acceptable imaging and measured on physical examination at 30° or more of flexion (but less than 45°) measured from the vertical position (zero degrees), and involvement of two or more organs/body systems with one of the organs/body systems involved to at least a moderate level of severity.
orD. Repeated manifestations of inflammatory arthritis, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.10 Sjögren's syndrome. As described in 14.00D7. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. Repeated manifestations of Sjögren's syndrome, with at least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
14.11 Human immunodeficiency virus (HIV) infection. With documentation as described in 14.00F1 and one of the following:
A. Multicentric (not localized or unicentric) Castleman disease affecting multiple groups of lymph nodes or organs containing lymphoid tissue (see 14.00F3a).
ORB. Primary central nervous system lymphoma (see 14.00F3b).
ORC. Primary effusion lymphoma (see 14.00F3c).
ORD. Progressive multifocal leukoencephalopathy (see 14.00F3d).
ORE. Pulmonary Kaposi sarcoma (see 14.00F3e).
ORF. Absolute CD4 count of 50 cells/mm 3 or less (see 14.00F4).
ORG. Absolute CD4 count of less than 200 cells/mm 3 or CD4 percentage of less than 14 percent, and one of the following (values do not have to be measured on the same date) (see 14.00F5):
1. BMI measurement of less than 18.5; or
2. Hemoglobin measurement of less than 8.0 grams per deciliter (g/dL).
ORH. Complication(s) of HIV infection requiring at least three hospitalizations within a 12-month period and at least 30 days apart (see 14.00F6). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
ORI. Repeated (as defined in 14.00I3) manifestations of HIV infection, including those listed in 14.11A-H, but without the requisite findings for those listings (for example, Kaposi sarcoma not meeting the criteria in 14.11E), or other manifestations (including, but not limited to, cardiovascular disease (including myocarditis, pericardial effusion, pericarditis, endocarditis, or pulmonary arteritis), diarrhea, distal sensory polyneuropathy, glucose intolerance, gynecologic conditions (including cervical cancer or pelvic inflammatory disease, see 14.00F7), hepatitis, HIV-associated dementia, immune reconstitution inflammatory syndrome (IRIS), infections (bacterial, fungal, parasitic, or viral), lipodystrophy (lipoatrophy or lipohypertrophy), malnutrition, muscle weakness, myositis, neurocognitive or other mental limitations not meeting the criteria in 12.00, oral hairy leukoplakia, osteoporosis, pancreatitis, peripheral neuropathy) resulting in significant, documented symptoms or signs (for example, but not limited to, fever, headaches, insomnia, involuntary weight loss, malaise, nausea, night sweats, pain, severe fatigue, or vomiting) and one of the following at the marked level:
1. Limitation of activities of daily living.
2. Limitation in maintaining social functioning.
3. Limitation in completing tasks in a timely manner due to deficiencies in concentration, persistence, or pace.
Part BMedical criteria for the evaluation of impairments of children under age 18 (where criteria in part A do not give appropriate consideration to the particular disease process in childhood).
Sec. 100.00 Low Birth Weight and Failure to Thrive. 101.00 Musculoskeletal System. 102.00 Special Senses and Speech. 103.00 Respiratory Disorders. 104.00 Cardiovascular System. 105.00 Digestive System. 106.00 Genitourinary Disorders. 107.00 Hematological Disorders. 108.00 Skin Disorders 109.00 Endocrine Disorders. 110.00 Congenital Disorders That Affect Multiple Body Systems. 111.00 Neurological Disorders. 112.00 Mental Disorders. 113.00 Cancer (Malignant Neoplastic Diseases) 114.00 Immune System Disorders. 100.00 Low Birth Weight and Failure to ThriveA. What conditions do we evaluate under these listings? We evaluate low birth weight (LBW) in infants from birth to attainment of age 1 and failure to thrive (FTT) in infants and toddlers from birth to attainment of age 3.
B. How do we evaluate disability based on LBW under 100.04? In 100.04A and 100.04B, we use an infant's birth weight as documented by an original or certified copy of the infant's birth certificate or by a medical record signed by a physician. Birth weight means the first weight recorded after birth. In 100.04B, gestational age is the infant's age based on the date of conception as recorded in the medical record. If the infant's impairment meets the requirements for listing 100.04A or 100.04B, we will follow the rule in § 416.990(b)(11) of this chapter.
C. How do we evaluate disability based on FTT under 100.05?
1. General. We establish FTT with or without a known cause when we have documentation of an infant's or a toddler's growth failure and developmental delay from an acceptable medical source(s) as defined in § 416.913(a) of this chapter. We require documentation of growth measurements in 100.05A and developmental delay described in 100.05B or 100.05C within the same consecutive 12-month period. The dates of developmental testing and reports may be different from the dates of growth measurements. After the attainment of age 3, we evaluate growth failure under the affected body system(s).
2. Growth failure. Under 100.05A, we use the appropriate table(s) under 105.08B in the digestive system to determine whether a child's growth is less than the third percentile. The child does not need to have a digestive disorder for purposes of 100.05.
a. For children from birth to attainment of age 2, we use the weight-for-length table corresponding to the child's gender (Table I or Table II).
b. For children age 2 to attainment of age 3, we use the body mass index (BMI)-for-age table corresponding to the child's gender (Table III or Table IV).
c. BMI is the ratio of a child's weight to the square of his or her height. We calculate BMI using the formulas in 105.00G2c.
d. Growth measurements. The weight-for-length measurements for children from birth to the attainment of age 2 and BMI-for-age measurements for children age 2 to attainment of age 3 that are required for this listing must be obtained within a 12-month period and at least 60 days apart. If a child attains age 2 during the evaluation period, additional measurements are not needed. Any measurements taken before the child attains age 2 can be used to evaluate the impairment under the appropriate listing for the child's age. If the child attains age 3 during the evaluation period, the measurements can be used to evaluate the impairment in the affected body system.
3. Developmental delay.
a. Under 100.05B and C, we use reports from acceptable medical sources to establish delay in a child's development.
b. Under 100.05B, we document the severity of developmental delay with results from a standardized developmental assessment, which compares a child's level of development to the level typically expected for his or her chronological age. If the child was born prematurely, we may use the corrected chronological age (CCA) for comparison. (See § 416.924b(b) of this chapter.) CCA is the chronological age adjusted by a period of gestational prematurity. CCA = (chronological age) - (number of weeks premature). Acceptable medical sources or early intervention specialists, physical or occupational therapists, and other sources may conduct standardized developmental assessments and developmental screenings. The results of these tests and screenings must be accompanied by a statement or records from an acceptable medical source who established the child has a developmental delay.
c. Under 100.05C, when there are no results from a standardized developmental assessment in the case record, we need narrative developmental reports from the child's medical sources in sufficient detail to assess the severity of his or her developmental delay. A narrative developmental report is based on clinical observations, progress notes, and well-baby check-ups. To meet the requirements for 100.05C, the report must include: The child's developmental history; examination findings (with abnormal findings noted on repeated examinations); and an overall assessment of the child's development (that is, more than one or two isolated skills) by the medical source. Some narrative developmental reports may include results from developmental screening tests, which can identify a child who is not developing or achieving skills within expected timeframes. Although medical sources may refer to screening test results as supporting evidence in the narrative developmental report, screening test results alone cannot establish a diagnosis or the severity of developmental delay.
D. How do we evaluate disorders that do not meet one of these listings?
1. We may find infants disabled due to other disorders when their birth weights are greater than 1200 grams but less than 2000 grams and their weight and gestational age do not meet listing 100.04. The most common disorders of prematurity and LBW include retinopathy of prematurity (ROP), chronic lung disease of infancy (CLD, previously known as bronchopulmonary dysplasia, or BPD), intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), and periventricular leukomalacia (PVL). Other disorders include poor nutrition and growth failure, hearing disorders, seizure disorders, cerebral palsy, and developmental disorders. We evaluate these disorders under the affected body systems.
2. We may evaluate infants and toddlers with growth failure that is associated with a known medical disorder under the body system of that medical disorder, for example, the respiratory or digestive body systems.
3. If an infant or toddler has a severe medically determinable impairment(s) that does not meet the criteria of any listing, we must also consider whether the child has an impairment(s) that medically equals a listing (see § 416.926 of this chapter). If the child's impairment(s) does not meet or medically equal a listing, we will determine whether the child's impairment(s) functionally equals the listings (see § 416.926a of this chapter) considering the factors in § 416.924a of this chapter. We use the rule in § 416.994a of this chapter when we decide whether a child continues to be disabled.
100.01 Category of Impairments, Low Birth Weight and Failure to Thrive
100.04 Low birth weight in infants from birth to attainment of age 1.
A. Birth weight (see 100.00B) of less than 1200 grams.
ORB. The following gestational age and birth weight:
| Gestational age (in weeks) |
Birth weight |
|---|---|
| 37-40 | 2000 grams or less. |
| 36 | 1875 grams or less. |
| 35 | 1700 grams or less. |
| 34 | 1500 grams or less. |
| 33 | 1325 grams or less. |
| 32 | 1250 grams or less. |
100.05 Failure to thrive in children from birth to attainment of age 3 (see 100.00C), documented by A and B, or A and C.
A. Growth failure as required in 1 or 2:
1. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate weight-for-length table in listing 105.08B1; or
2. For children age 2 to attainment of age 3, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate BMI-for-age table in listing 105.08B2.
ANDB. Developmental delay (see 100.00C1 and C3), established by an acceptable medical source and documented by findings from one current report of a standardized developmental assessment (see 100.00C3b) that:
1. Shows development not more than two-thirds of the level typically expected for the child's age; or
2. Results in a valid score that is at least two standard deviations below the mean.
ORC. Developmental delay (see 100.00C3), established by an acceptable medical source and documented by findings from two narrative developmental reports (see 100.00C3c) that:
1. Are dated at least 120 days apart (see 100.00C1); and
2. Indicate current development not more than two-thirds of the level typically expected for the child's age.
101.00 Musculoskeletal SystemA. Disorders of the musculoskeletal system may result from hereditary, congenital, or acquired pathologic processes. Impairments may result from infectious, inflammatory, or degenerative processes, traumatic or developmental events, or neoplastic, vascular, or toxic/metabolic diseases.
B. Loss of Function1. General. Under this section, loss of function may be due to bone or joint deformity or destruction from any cause; miscellaneous disorders of the spine with or without radiculopathy or other neurological deficits; amputation; or fractures or soft tissue injuries, including burns, requiring prolonged periods of immobility or convalescence. The provisions of 101.02 and 101.03 notwithstanding, inflammatory arthritis is evaluated under 114.09 (see 114.00D6). We evaluate impairments with neurological causes under 111.00, as appropriate.
2. How We Define Loss of Function in These Listingsa. General. Regardless of the cause(s) of a musculoskeletal impairment, functional loss for purposes of these listings is defined as the inability to ambulate effectively on a sustained basis for any reason, including pain associated with the underlying musculoskeletal impairment, or the inability to perform fine and gross movements effectively on a sustained basis for any reason, including pain associated with the underlying musculoskeletal impairment. The inability to ambulate effectively or the inability to perform fine and gross movements effectively must have lasted, or be expected to last, for at least 12 months. For the purposes of these criteria, consideration of the ability to perform these activities must be from a physical standpoint alone. When there is an inability to perform these activities due to a mental impairment, the criteria in 112.00ff are to be used. We will determine whether a child can ambulate effectively or can perform fine and gross movements effectively based on the medical and other evidence in the case record, generally without developing additional evidence about the child's ability to perform the specific activities listed as examples in 101.00B2b(2) and (3) and 101.00B2c(2) and (3).
b. What We Mean by Inability To Ambulate Effectively(1) Definition. Inability to ambulate effectively means an extreme limitation of the ability to walk; i.e., an impairment that interferes very seriously with the child's ability to independently initiate, sustain, or complete activities. Ineffective ambulation is defined generally as having insufficient lower extremity functioning (see 101.00J) to permit independent ambulation without the use of a hand-held assistive device(s) that limits the functioning of both upper extremities. (Listing 101.05C is an exception to this general definition because the child has the use of only one upper extremity due to amputation of a hand.)
(2) How we assess inability to ambulate effectively for children too young to be expected to walk independently. For children who are too young to be expected to walk independently, consideration of function must be based on assessment of limitations in the ability to perform comparable age-appropriate activities with the lower extremities, given normal developmental expectations. For such children, an extreme level of limitation means skills or performance at no greater than one-half of age-appropriate expectations based on an overall developmental assessment rather than on one or two isolated skills.
(3) How we assess inability to ambulate effectively for older children. Older children, who would be expected to be able to walk when compared to other children the same age who do not have impairments, must be capable of sustaining a reasonable walking pace over a sufficient distance to be able to carry out age-appropriate activities. They must have the ability to travel age-appropriately without extraordinary assistance to and from school or a place of employment. Therefore, examples of ineffective ambulation for older children include, but are not limited to, the inability to walk without the use of a walker, two crutches or two canes, the inability to walk a block at a reasonable pace on rough or uneven surfaces, the inability to use standard public transportation, the inability to carry out age-appropriate school activities independently, and the inability to climb a few steps at a reasonable pace with the use of a single hand rail. The ability to walk independently about the child's home or a short distance at school without the use of assistive devices does not, in and of itself, constitute effective ambulation.
c. What We Mean by Inability To Perform Fine and Gross Movements Effectively(1) Definition. Inability to perform fine and gross movements effectively means an extreme loss of function of both upper extremities; i.e., an impairment that interferes very seriously with the child's ability to independently initiate, sustain, or complete activities. To use their upper extremities effectively, a child must be capable of sustaining such functions as reaching, pushing, pulling, grasping, and fingering in an age-appropriate manner to be able to carry out age-appropriate activities.
(2) How we assess inability to perform fine and gross movements in very young children. For very young children, we consider limitations in the ability to perform comparable age-appropriate activities involving the upper extremities compared to the ability of children the same age who do not have impairments. For such children, an extreme level of limitation means skills or performance at no greater than one-half of age-appropriate expectations based on an overall developmental assessment.
(3) How we assess inability to perform fine and gross movements in older children. For older children, examples of inability to perform fine and gross movements effectively include, but are not limited to, the inability to prepare a simple meal and feed oneself, the inability to take care of personal hygiene, or the inability to sort and handle papers or files, depending upon which activities are age-appropriate.
d. Pain or other symptoms. Pain or other symptoms may be an important factor contributing to functional loss. In order for pain or other symptoms to be found to affect a child's ability to function in an age-appropriate manner or to perform basic work activities, medical signs or laboratory findings must show the existence of a medically determinable impairment(s) that could reasonably be expected to produce the pain or other symptoms. The musculoskeletal listings that include pain or other symptoms among their criteria also include criteria for limitations in functioning as a result of the listed impairment, including limitations caused by pain. It is, therefore, important to evaluate the intensity and persistence of such pain or other symptoms carefully in order to determine their impact on the child's functioning under these listings. See also §§ 404.1525(f) and 404.1529 of this part, and §§ 416.925(f) and 416.929 of part 416 of this chapter.
C. Diagnosis and Evaluation1. General. Diagnosis and evaluation of musculoskeletal impairments should be supported, as applicable, by detailed descriptions of the joints, including ranges of motion, condition of the musculature (e.g., weakness, atrophy), sensory or reflex changes, circulatory deficits, and laboratory findings, including findings on x-ray or other appropriate medically acceptable imaging. Medically acceptable imaging includes, but is not limited to, x-ray imaging, computerized axial tomography (CAT scan) or magnetic resonance imaging (MRI), with or without contrast material, myelography, and radionuclear bone scans. “Appropriate” means that the technique used is the proper one to support the evaluation and diagnosis of the impairment.
2. Purchase of certain medically acceptable imaging. While any appropriate medically acceptable imaging is useful in establishing the diagnosis of musculoskeletal impairments, some tests, such as CAT scans and MRIs, are quite expensive, and we will not routinely purchase them. Some, such as myelograms, are invasive and may involve significant risk. We will not order such tests. However, when the results of any of these tests are part of the existing evidence in the case record we will consider them together with the other relevant evidence.
3. Consideration of electrodiagnostic procedures. Electrodiagnostic procedures may be useful in establishing the clinical diagnosis, but do not constitute alternative criteria to the requirements of 101.04.
D. The physical examination must include a detailed description of the rheumatological, orthopedic, neurological, and other findings appropriate to the specific impairment being evaluated. These physical findings must be determined on the basis of objective observation during the examination and not simply a report of the child's allegation; e.g., “He says his leg is weak, numb.” Alternative testing methods should be used to verify the abnormal findings; e.g., a seated straight-leg raising test in addition to a supine straight-leg raising test. Because abnormal physical findings may be intermittent, their presence over a period of time must be established by a record of ongoing management and evaluation. Care must be taken to ascertain that the reported examination findings are consistent with the child's age and activities.
E. Examination of the Spine1. General. Examination of the spine should include a detailed description of gait, range of motion of the spine given quantitatively in degrees from the vertical position (zero degrees) or, for straight-leg raising from the sitting and supine position (zero degrees), any other appropriate tension signs, motor and sensory abnormalities, muscle spasm, when present, and deep tendon reflexes. Observations of the child during the examination should be reported; e.g., how he or she gets on and off the examination table. Inability to walk on the heels or toes, to squat, or to arise from a squatting position, when appropriate, may be considered evidence of significant motor loss. However, a report of atrophy is not acceptable as evidence of significant motor loss without circumferential measurements of both thighs and lower legs, or both upper and lower arms, as appropriate, at a stated point above and below the knee or elbow given in inches or centimeters. Additionally, a report of atrophy should be accompanied by measurement of the strength of the muscle(s) in question generally based on a grading system of 0 to 5, with 0 being complete loss of strength and 5 being maximum strength. A specific description of atrophy of hand muscles is acceptable without measurements of atrophy but should include measurements of grip and pinch strength. However, because of the unreliability of such measurement in younger children, these data are not applicable to children under 5 years of age.
2. When neurological abnormalities persist. Neurological abnormalities may not completely subside after treatment or with the passage of time. Therefore, residual neurological abnormalities that persist after it has been determined clinically or by direct surgical or other observation that the ongoing or progressive condition is no longer present will not satisfy the required findings in 101.04. More serious neurological deficits (paraparesis, paraplegia) are to be evaluated under the criteria in 111.00ff.
F. Major joints refers to the major peripheral joints, which are the hip, knee, shoulder, elbow, wrist-hand, and ankle-foot, as opposed to other peripheral joints (e.g., the joints of the hand or forefoot) or axial joints (i.e., the joints of the spine.) The wrist and hand are considered together as one major joint, as are the ankle and foot. Since only the ankle joint, which consists of the juncture of the bones of the lower leg (tibia and fibula) with the hindfoot (tarsal bones), but not the forefoot, is crucial to weight bearing, the ankle and foot are considered separately in evaluating weight bearing.
G. Measurements of joint motion are based on the techniques described in the chapter on the extremities, spine, and pelvis in the current edition of the “Guides to the Evaluation of Permanent Impairment” published by the American Medical Association.
H. Documentation.1. General. Musculoskeletal impairments frequently improve with time or respond to treatment. Therefore, a longitudinal clinical record is generally important for the assessment of severity and expected duration of an impairment unless the child is a newborn or the claim can be decided favorably on the basis of the current evidence.
2. Documentation of medically prescribed treatment and response. Many children, especially those who have listing-level impairments, will have received the benefit of medically prescribed treatment. Whenever evidence of such treatment is available it must be considered.
3. When there is no record of ongoing treatment. Some children will not have received ongoing treatment or have an ongoing relationship with the medical community despite the existence of a severe impairment(s). In such cases, evaluation will be made on the basis of the current objective medical evidence and other available evidence, taking into consideration the child's medical history, symptoms, and medical source opinions. Even though a child who does not receive treatment may not be able to show an impairment that meets the criteria of one of the musculoskeletal listings, the child may have an impairment(s) that is either medically or, in the case of a claim for benefits under part 416 of this chapter, functionally equivalent in severity to one of the listed impairments.
4. Evaluation when the criteria of a musculoskeletal listing are not met. These listings are only examples of common musculoskeletal disorders that are severe enough to find a child disabled. Therefore, in any case in which a child has a medically determinable impairment that is not listed, an impairment that does not meet the requirements of a listing, or a combination of impairments no one of which meets the requirements of a listing, we will consider whether the child's impairment(s) is medically or, in the case of a claim for benefits under part 416 of this chapter, functionally equivalent in severity to the criteria of a listing. (See §§ 404.1526, 416.926, and 416.926a.) Individuals with claims for benefits under part 404, who have an impairment(s) with a level of severity that does not meet or equal the criteria of the musculoskeletal listings may or may not have the RFC that would enable them to engage in substantial gainful activity. Evaluation of the impairment(s) of these individuals should proceed through the final steps of the sequential evaluation process in § 404.1520 (or, as appropriate, the steps in the medical improvement review standard in § 404.1594).
I. Effects of Treatment1. General. Treatments for musculoskeletal disorders may have beneficial effects or adverse side effects. Therefore, medical treatment (including surgical treatment) must be considered in terms of its effectiveness in ameliorating the signs, symptoms, and laboratory abnormalities of the disorder, and in terms of any side effects that may further limit the child.
2. Response to treatment. Response to treatment and adverse consequences of treatment may vary widely. For example, a pain medication may relieve a child's pain completely, partially, or not at all. It may also result in adverse effects, e.g., drowsiness, dizziness, or disorientation, that compromise the child's ability to function. Therefore, each case must be considered on an individual basis, and include consideration of the effects of treatment on the child's ability to function.
3. Documentation. A specific description of the drugs or treatment given (including surgery), dosage, frequency of administration, and a description of the complications or response to treatment should be obtained. The effects of treatment may be temporary or long-term. As such, the finding regarding the impact of treatment must be based on a sufficient period of treatment to permit proper consideration or judgment about future functioning.
J. Orthotic, Prosthetic, or Assistive Devices1. General. Consistent with clinical practice, children with musculoskeletal impairments may be examined with and without the use of any orthotic, prosthetic, or assistive devices as explained in this section.
2. Orthotic devices. Examination should be with the orthotic device in place and should include an evaluation of the child's maximum ability to function effectively with the orthosis. It is unnecessary to routinely evaluate the child's ability to function without the orthosis in place. If the child has difficulty with, or is unable to use, the orthotic device, the medical basis for the difficulty should be documented. In such cases, if the impairment involves a lower extremity or extremities, the examination should include information on the child's ability to ambulate effectively without the device in place unless contraindicated by the medical judgment of a physician who has treated or examined the child.
3. Prosthetic devices. Examination should be with the prosthetic device in place. In amputations involving a lower extremity or extremities, it is unnecessary to evaluate the child's ability to walk without the prosthesis in place. However, the child's medical ability to use a prosthesis to ambulate effectively, as defined in 101.00B2b, should be evaluated. The condition of the stump should be evaluated without the prosthesis in place.
4. Hand-held assistive devices. When a child with an impairment involving a lower extremity or extremities uses a hand-held assistive device, such as a cane, crutch or walker, examination should be with and without the use of the assistive device unless contraindicated by the medical judgment of a physician who has treated or examined the child. The child's ability to ambulate with and without the device provides information as to whether, or the extent to which, the child is able to ambulate without assistance. The medical basis for the use of any assistive device (e.g., instability, weakness) should be documented. The requirement to use a hand-held assistive device may also impact on the child's functional capacity by virtue of the fact that one or both upper extremities are not available for such activities as lifting, carrying, pushing, and pulling.
K. Disorders of the spine, listed in 101.04, result in limitations because of distortion of the bony and ligamentous architecture of the spine and associated impingement on nerve roots (including the cauda equina) or spinal cord. Such impingement on nerve tissue may result from a herniated nucleus pulposus, spinal stenosis, arachnoiditis, or other miscellaneous conditions.
L. Abnormal curvatures of the spine. Abnormal curvatures of the spine (specifically, scoliosis, kyphosis and kyphoscoliosis) can result in impaired ambulation, but may also adversely affect functioning in body systems other than the musculoskeletal system. For example, a child's ability to breathe may be affected; there may be cardiac difficulties (e.g., impaired myocardial function); or there may be disfigurement resulting in withdrawal or isolation. When there is impaired ambulation, evaluation of equivalence may be made by reference to 114.09A. When the abnormal curvature of the spine results in symptoms related to fixation of the dorsolumbar or cervical spine, evaluation of equivalence may be made by reference to 114.09C. When there is respiratory or cardiac involvement or an associated mental disorder, evaluation may be made under 103.00ff, 104.00ff, or 112.00ff, as appropriate. Other consequences should be evaluated according to the listing for the affected body system.
M. Under continuing surgical management, as used in 101.07 and 101.08, refers to surgical procedures and any other associated treatments related to the efforts directed toward the salvage or restoration of functional use of the affected part. It may include such factors as post-surgical procedures, surgical complications, infections, or other medical complications, related illnesses, or related treatments that delay the child's attainment of maximum benefit from therapy. When burns are not under continuing surgical management, see 108.00F.
N. After maximum benefit from therapy has been achieved in situations involving fractures of an upper extremity (101.07), or soft tissue injuries (101.08), i.e., there have been no significant changes in physical findings or on appropriate medically acceptable imaging for any 6-month period after the last definitive surgical procedure or other medical intervention, evaluation must be made on the basis of the demonstrable residuals, if any. A finding that 101.07 or 101.08 is met must be based on a consideration of the symptoms, signs, and laboratory findings associated with recent or anticipated surgical procedures and the resulting recuperative periods, including any related medical complications, such as infections, illnesses, and therapies which impede or delay the efforts toward restoration of function. Generally, when there has been no surgical or medical intervention for 6 months after the last definitive surgical procedure, it can be concluded that maximum therapeutic benefit has been reached. Evaluation at this point must be made on the basis of the demonstrable residual limitations, if any, considering the child's impairment-related symptoms, signs, and laboratory findings, any residual symptoms, signs, and laboratory findings associated with such surgeries, complications, and recuperative periods, and other relevant evidence.
O. Major function of the face and head, for purposes of listing 101.08, relates to impact on any or all of the activities involving vision, hearing, speech, mastication, and the initiation of the digestive process.
P. When surgical procedures have been performed, documentation should include a copy of the operative notes and available pathology reports.
101.01 Category of Impairments, Musculoskeletal101.02 Major dysfunction of a joint(s) (due to any cause): Characterized by gross anatomical deformity (e.g., subluxation, contracture, bony or fibrous ankylosis, instability) and chronic joint pain and stiffness with signs of limitation of motion or other abnormal motion of the affected joint(s), and findings on appropriate medically acceptable imaging of joint space narrowing, bony destruction, or ankylosis of the affected joint(s). With:
A. Involvement of one major peripheral weight-bearing joint (i.e., hip, knee, or ankle), resulting in inability to ambulate effectively, as defined in 101.00B2b;
orB. Involvement of one major peripheral joint in each upper extremity (i.e., shoulder, elbow, or wrist-hand), resulting in inability to perform fine and gross movements effectively, as defined in 101.00B2c.
101.03 Reconstructive surgery or surgical arthrodesis of a major weight-bearing joint, with inability to ambulate effectively, as defined in 101.00B2b, and return to effective ambulation did not occur, or is not expected to occur, within 12 months of onset.
101.04 Disorders of the spine (e.g., lysosomal disorders, metabolic disorders, vertebral osteomyelitis, vertebral fracture, achondroplasia) resulting in compromise of a nerve root (including the cauda equina) or the spinal cord, with evidence of nerve root compression characterized by neuro-anatomic distribution of pain, limitation of motion of the spine, motor loss (atrophy with associated muscle weakness or muscle weakness) accompanied by sensory or reflex loss and, if there is involvement of the lower back, positive straight-leg raising test (sitting and supine).
101.05 Amputation (due to any cause).
A. Both hands;
orB. One or both lower extremities at or above the tarsal region, with stump complications resulting in medical inability to use a prosthetic device to ambulate effectively, as defined in 101.00B2b, which have lasted or are expected to last for at least 12 months;
orC. One hand and one lower extremity at or above the tarsal region, with inability to ambulate effectively, as defined in 101.00B2b;
orD. Hemipelvectomy or hip disarticulation.
101.06 Fracture of the femur, tibia, pelvis, or one or more of the tarsal bones. With:
A. Solid union not evident on appropriate medically acceptable imaging, and not clinically solid;
andB. Inability to ambulate effectively, as defined in 101.00B2b, and return to effective ambulation did not occur or is not expected to occur within 12 months of onset.
101.07 Fracture of an upper extremity with nonunion of a fracture of the shaft of the humerus, radius, or ulna, under continuing surgical management, as defined in 101.00M, directed toward restoration of functional use of the extremity, and such function was not restored or expected to be restored within 12 months of onset.
101.08 Soft tissue injury (e.g., burns) of an upper or lower extremity, trunk, or face and head, under continuing surgical management, as defined in 101.00M, directed toward the salvage or restoration of major function, and such major function was not restored or expected to be restored within 12 months of onset. Major function of the face and head is described in 101.00O.
102.00 Special Senses and SpeechA. How do we evaluate visual disorders?
1. What are visual disorders? Visual disorders are abnormalities of the eye, the optic nerve, the optic tracts, or the brain that may cause a loss of visual acuity or visual fields. A loss of visual acuity limits your ability to distinguish detail, read, do fine work, or perform other age-appropriate activities. A loss of visual fields limits your ability to perceive visual stimuli in the peripheral extent of vision.
2. How do we define statutory blindness? Statutory blindness is blindness as defined in sections 216(i)(1) and 1614(a)(2) of the Social Security Act (Act).
a. The Act defines blindness as central visual acuity of 20/200 or less in the better eye with the use of a correcting lens. We use your best-corrected central visual acuity for distance in the better eye when we determine if this definition is met. (For visual acuity testing requirements, see 102.00A5.)
b. The Act also provides that an eye that has a visual field limitation such that the widest diameter of the visual field subtends an angle no greater than 20 degrees is considered as having a central visual acuity of 20/200 or less. (For visual field testing requirements, see 102.00A6.)
c. You have statutory blindness only if your visual disorder meets the criteria of 102.02A, 102.02B, or 102.03A. You do not have statutory blindness if your visual disorder medically equals the criteria of 102.02A, 102.02B, or 102.03A or meets or medically equals the criteria of 102.03B, 102.03C, 102.04A, or 102.04B because your disability is based on criteria other than those in the statutory definition of blindness.
3. What evidence do we need to establish statutory blindness under title XVI? To establish that you have statutory blindness under title XVI, we need evidence showing only that your central visual acuity in your better eye or your visual field in your better eye meets the criteria in 102.00A2, provided that those measurements are consistent with the other evidence in your case record. We do not need documentation of the cause of your blindness. Also, there is no duration requirement for statutory blindness under title XVI (see §§ 416.981 and 416.983 of this chapter).
4. What evidence do we need to evaluate visual disorders, including those that result in statutory blindness under title II? To evaluate your visual disorder, we usually need a report of an eye examination that includes measurements of your best-corrected central visual acuity (see 102.00A5) or the extent of your visual fields (see 102.00A6), as appropriate. If you have visual acuity or visual field loss, we need documentation of the cause of the loss. A standard eye examination will usually indicate the cause of any visual acuity loss. A standard eye examination can also indicate the cause of some types of visual field deficits. Some disorders, such as cortical visual disorders, may result in abnormalities that do not appear on a standard eye examination. If the standard eye examination does not indicate the cause of your vision loss, we will request the information used to establish the presence of your visual disorder. If your visual disorder does not satisfy the criteria in 102.02, 102.03, or 102.04, we will request a description of how your visual disorder affects your ability to function.
5. How do we measure your best-corrected central visual acuity?
a. Visual acuity testing. When we need to measure your best-corrected central visual acuity, which is your optimal visual acuity attainable with the use of a corrective lens, we use visual acuity testing for distance that was carried out using Snellen methodology or any other testing methodology that is comparable to Snellen methodology.
(i) Your best-corrected central visual acuity for distance is usually measured by determining what you can see from 20 feet. If your visual acuity is measured for a distance other than 20 feet, we will convert it to a 20-foot measurement. For example, if your visual acuity is measured at 10 feet and is reported as 10/40, we will convert this measurement to 20/80.
(ii) A visual acuity recorded as CF (counts fingers), HM (hand motion only), LP or LPO (light perception or light perception only), or NLP (no light perception) indicates that no optical correction will improve your visual acuity. If your central visual acuity in an eye is recorded as CF, HM, LP or LPO, or NLP, we will determine that your best-corrected central visual acuity is 20/200 or less in that eye.
(iii) We will not use the results of pinhole testing or automated refraction acuity to determine your best-corrected central visual acuity. These tests provide an estimate of potential visual acuity but not an actual measurement of your best-corrected central visual acuity.
(iv) Very young children, such as infants and toddlers, cannot participate in testing using Snellen methodology or other comparable testing. If you are unable to participate in testing using Snellen methodology or other comparable testing due to your young age, we will consider clinical findings of your fixation and visual-following behavior. If both these behaviors are absent, we will consider the anatomical findings or the results of neuroimaging, electroretinogram, or visual evoked response (VER) testing when this testing has been performed.
b. Other test charts.
(i) Children between the ages of 3 and 5 often cannot identify the letters on a Snellen or other letter test chart. Specialists with expertise in assessment of childhood vision use alternate methods for measuring visual acuity in young children. We consider alternate methods, for example, the Landolt C test or the tumbling-E test, which are used to evaluate young children who are unable to participate in testing using Snellen methodology, to be comparable to testing using Snellen methodology.
(ii) Most test charts that use Snellen methodology do not have lines that measure visual acuity between 20/100 and 20/200. Some test charts, such as the Bailey-Lovie or the Early Treatment Diabetic Retinopathy Study (ETDRS), used mostly in research settings, have such lines. If your visual acuity is measured with one of these charts, and you cannot read any of the letters on the 20/100 line, we will determine that you have statutory blindness based on a visual acuity of 20/200 or less. For example, if your best-corrected central visual acuity for distance in the better eye is 20/160 using an ETDRS chart, we will find that you have statutory blindness. Regardless of the type of test chart used, you do not have statutory blindness if you can read at least one letter on the 20/100 line. For example, if your best-corrected central visual acuity for distance in the better eye is 20/125 + 1 using an ETDRS chart, we will find that you do not have statutory blindness because you are able to read one letter on the 20/100 line.
c. Testing using a specialized lens. In some instances, you may have visual acuity testing performed using a specialized lens, such as a contact lens. We will use the visual acuity measurements obtained with a specialized lens only if you have demonstrated the ability to use the specialized lens on a sustained basis. We will not use visual acuity measurements obtained with telescopic lenses.
d. Cycloplegic refraction is an examination of the eye performed after administering cycloplegic eye drops capable of relaxing the ability of the pupil to become smaller and temporarily paralyzing the focusing muscles. If your case record contains the results of cycloplegic refraction, we may use the results to determine your best-corrected central visual acuity. We will not purchase cycloplegic refraction.
e. VER testing measures your response to visual events and can often detect dysfunction that is undetectable through other types of examinations. If you have an absent response to VER testing in your better eye, we will determine that your best-corrected central visual acuity is 20/200 or less in that eye and that your visual acuity loss satisfies the criterion in 102.02A or 102.02B4, as appropriate, when these test results are consistent with the other evidence in your case record. If you have a positive response to VER testing in an eye, we will not use that result to determine your best-corrected central visual acuity in that eye.
6. How do we measure your visual fields?
a. General. We generally need visual field testing when you have a visual disorder that could result in visual field loss, such as glaucoma, retinitis pigmentosa, or optic neuropathy, or when you display behaviors that suggest a visual field loss. When we need to measure the extent of your visual field loss, we use visual field testing (also referred to as perimetry) carried out using automated static threshold perimetry performed on an acceptable perimeter. (For perimeter requirements, see 102.00A9.)
b. Automated static threshold perimetry requirements.
(i) The test must use a white size III Goldmann stimulus and a 31.5 apostilb (asb) white background (or a 10 candela per square meter (cd/m 2) white background). The stimuli test locations must be no more than 6 degrees apart horizontally or vertically. Measurements must be reported on standard charts and include a description of the size and intensity of the test stimulus.
(ii) We measure the extent of your visual field loss by determining the portion of the visual field in which you can see a white III4e stimulus. The “III” refers to the standard Goldmann test stimulus size III (4 mm 2), and the “4e” refers to the standard Goldmann intensity filter (0 decibel (dB) attenuation, which allows presentation of the maximum luminance) used to determine the intensity of the stimulus.
(iii) In automated static threshold perimetry, the intensity of the stimulus varies. The intensity of the stimulus is expressed in decibels (dB). A perimeter's maximum stimulus luminance is usually assigned the value 0 dB. We need to determine the dB level that corresponds to a 4e intensity for the particular perimeter being used. We will then use the dB printout to determine which points you see at a 4e intensity level (a “seeing point”). For example:
A. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 10,000 asb, a 10 dB stimulus is equivalent to a 4e stimulus. Any point you see at 10 dB or greater is a seeing point.
B. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 4,000 asb, a 6 dB stimulus is equivalent to a 4e stimulus. Any point you see at 6 dB or greater is a seeing point.
C. When the maximum stimulus luminance (0 dB stimulus) on an acceptable perimeter is 1,000 asb, a 0 dB stimulus is equivalent to a 4e stimulus. Any point you see at 0 dB or greater is a seeing point.
c. Evaluation under 102.03A. To determine statutory blindness based on visual field loss in your better eye (102.03A), we need the results of a visual field test that measures the central 24 to 30 degrees of your visual field; that is, the area measuring 24 to 30 degrees from the point of fixation. Acceptable tests include the Humphrey Field Analyzer (HFA) 30-2, HFA 24-2, and Octopus 32.
d. Evaluation under 102.03B. To determine whether your visual field loss meets listing 102.03B, we use the mean deviation or defect (MD) from acceptable automated static threshold perimetry that measures the central 30 degrees of the visual field. MD is the average sensitivity deviation from normal values for all measured visual field locations. When using results from HFA tests, which report the MD as a negative number, we use the absolute value of the MD to determine whether your visual field loss meets listing 102.03B. We cannot use tests that do not measure the central 30 degrees of the visual field, such as the HFA 24-2, to determine if your impairment meets or medically equals 102.03B.
e. Other types of perimetry. If your case record contains visual field measurements obtained using manual or automated kinetic perimetry, such as Goldmann perimetry or the HFA “SSA Test Kinetic,” we can generally use these results if the kinetic test was performed using a white III4e stimulus projected on a white 31.5 asb (10 cd/m 2) background. Automated kinetic perimetry, such as the HFA “SSA Test Kinetic,” does not detect limitations in the central visual field because testing along a meridian stops when you see the stimulus. If your visual disorder has progressed to the point at which it is likely to result in a significant limitation in the central visual field, such as a scotoma (see 102.00A6h), we will not use automated kinetic perimetry to determine the extent of your visual field loss. Instead, we will determine the extent of your visual field loss using automated static threshold perimetry or manual kinetic perimetry.
f. Screening tests. We will not use the results of visual field screening tests, such as confrontation tests, tangent screen tests, or automated static screening tests, to determine that your impairment meets or medically equals a listing, or functionally equals the listings. We can consider normal results from visual field screening tests to determine whether your visual disorder is severe when these test results are consistent with the other evidence in your case record. (See § 416.924(c) of this chapter.) We will not consider normal test results to be consistent with the other evidence if the clinical findings indicate that your visual disorder has progressed to the point that it is likely to cause visual field loss, or you have a history of an operative procedure for retinal detachment.
g. Use of corrective lenses. You must not wear eyeglasses during visual field testing because they limit your field of vision. You may wear contact lenses to correct your visual acuity during the visual field test to obtain the most accurate visual field measurements. For this single purpose, you do not need to demonstrate that you have the ability to use the contact lenses on a sustained basis.
h. Scotoma. A scotoma is a field defect or non-seeing area (also referred to as a “blind spot”) in the visual field surrounded by a normal field or seeing area. When we measure your visual field, we subtract the length of any scotoma, other than the normal blind spot, from the overall length of any diameter on which it falls.
7. How do we determine your visual acuity efficiency, visual field efficiency, and visual efficiency?
a. General. Visual efficiency, a calculated value of your remaining visual function, is the combination of your visual acuity efficiency and your visual field efficiency expressed as a percentage.
b. Visual acuity efficiency. Visual acuity efficiency is a percentage that corresponds to the best-corrected central visual acuity for distance in your better eye. See Table 1.
Table 1 - Visual Acuity Efficiency
| Snellen best-corrected central visual acuity for distance | Visual acuity
efficiency (%) (102.04A) |
|
|---|---|---|
| English | Metric | |
| 20/16 | 6/5 | 100 |
| 20/20 | 6/6 | 100 |
| 20/25 | 6/7.5 | 95 |
| 20/30 | 6/9 | 90 |
| 20/40 | 6/12 | 85 |
| 20/50 | 6/15 | 75 |
| 20/60 | 6/18 | 70 |
| 20/70 | 6/21 | 65 |
| 20/80 | 6/24 | 60 |
| 20/100 | 6/30 | 50 |
c. Visual field efficiency. Visual field efficiency is a percentage that corresponds to the visual field in your better eye. Under 102.03C, we require kinetic perimetry to determine your visual field efficiency percentage. We calculate the visual field efficiency percentage by adding the number of degrees you see along the eight principal meridians found on a visual field chart (0, 45, 90, 135, 180, 225, 270, and 315) in your better eye and dividing by 5. For example, in Figure 1:
A. The diagram of the left eye illustrates a visual field, as measured with a III4e stimulus, contracted to 30 degrees in two meridians (180 and 225) and to 20 degrees in the remaining six meridians. The visual efficiency percentage of this field is: ((2 × 30) + (6 × 20)) ÷ 5 = 36 percent.
B. The diagram of the right eye illustrates the extent of a normal visual field as measured with a III4e stimulus. The sum of the eight principal meridians of this field is 500 degrees. The visual efficiency percentage of this field is 500 ÷ 5 = 100 percent.
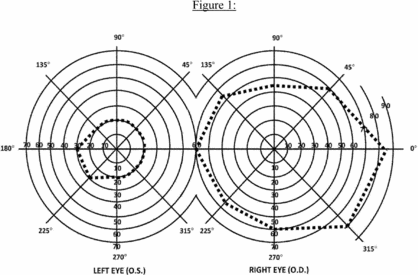
d. Visual efficiency. Under 102.04A, we calculate the visual efficiency percentage by multiplying your visual acuity efficiency percentage (see 102.00A7b) by your visual field efficiency percentage (see 102.00A7c) and dividing by 100. For example, if your visual acuity efficiency percentage is 75 and your visual field efficiency percentage is 36, your visual efficiency percentage is: (75 × 36) ÷ 100 = 27 percent.
8. How do we determine your visual acuity impairment value, visual field impairment value, and visual impairment value?
a. General. Visual impairment value, a calculated value of your loss of visual function, is the combination of your visual acuity impairment value and your visual field impairment value.
b. Visual acuity impairment value. Your visual acuity impairment value corresponds to the best-corrected central visual acuity for distance in your better eye. See Table 2.
Table 2 - Visual Acuity Impairment Value
| Snellen best-corrected central visual acuity for distance | Visual acuity impairment value (102.04B) |
|
|---|---|---|
| English | Metric | |
| 20/16 | 6/5 | 0.00 |
| 20/20 | 6/6 | 0.00 |
| 20/25 | 6/7.5 | 0.10 |
| 20/30 | 6/9 | 0.18 |
| 20/40 | 6/12 | 0.30 |
| 20/50 | 6/15 | 0.40 |
| 20/60 | 6/18 | 0.48 |
| 20/70 | 6/21 | 0.54 |
| 20/80 | 6/24 | 0.60 |
| 20/100 | 6/30 | 0.70 |
c. Visual field impairment value. Your visual field impairment value corresponds to the visual field in your better eye. Using the MD from acceptable automated static threshold perimetry, we calculate the visual field impairment value by dividing the absolute value of the MD by 22. For example, if your MD on an HFA 30-2 is −16, your visual field impairment value is: |−16| ÷ 22 = 0.73.
d. Visual impairment value. Under 102.04B, we calculate the visual impairment value by adding your visual acuity impairment value (see 102.00A8b) and your visual field impairment value (see 102.00A8c). For example, if your visual acuity impairment value is 0.48 and your visual field impairment value is 0.73, your visual impairment value is: 0.48 + 0.73 = 1.21.
9. What are our requirements for an acceptable perimeter? We will use results from automated static threshold perimetry performed on a perimeter that:
a. Uses optical projection to generate the test stimuli.
b. Has an internal normative database for automatically comparing your performance with that of the general population.
c. Has a statistical analysis package that is able to calculate visual field indices, particularly mean deviation or mean defect.
d. Demonstrates the ability to correctly detect visual field loss and correctly identify normal visual fields.
e. Demonstrates good test-retest reliability.
f. Has undergone clinical validation studies by three or more independent laboratories with results published in peer-reviewed ophthalmic journals.
B. How do we evaluate hearing loss?
1. What evidence do we need?
a. We need evidence showing that you have a medically determinable impairment that causes your hearing loss and audiometric measurements of the severity of your hearing loss. We generally require both an otologic examination and audiometric testing to establish that you have a medically determinable impairment that causes your hearing loss. You should have this audiometric testing within 2 months of the otologic examination. Once we have evidence that you have a medically determinable impairment, we can use the results of later audiometric testing to assess the severity of your hearing loss without another otologic examination. We will consider your test scores together with any other relevant information we have about your hearing, including information from outside of the test setting.
b. The otologic examination must be performed by a licensed physician (medical or osteopathic doctor) or audiologist. It must include your medical history, your description of how your hearing loss affects you, and the physician's or audiologist's description of the appearance of the external ears (pinnae and external ear canals), evaluation of the tympanic membranes, and assessment of any middle ear abnormalities.
c. Audiometric testing must be performed by, or under the direct supervision of, a licensed audiologist or an otolaryngologist.
2. What audiometric testing do we need when you do not have a cochlear implant?
a. General. We need either physiologic or behavioral testing (other than screening testing, see 102.00B2g) that is appropriate for your age at the time of testing. See 102.00B2c-102.00B2f. We will make every reasonable effort to obtain the results of physiologic testing that has been done; however, we will not purchase such testing.
b. Testing requirements. The testing must be conducted in accordance with the most recently published standards of the American National Standards Institute (ANSI). You must not wear hearing aids during the testing. Additionally, a person described in 102.00B1c must perform an otoscopic examination immediately before the audiometric testing. (An otoscopic examination provides a description of the appearance of your external ear canals and an evaluation of the tympanic membranes. In these rules, we use the term to include otoscopic examinations performed by physicians and otoscopic inspections performed by audiologists and others.) The otoscopic examination must show that there are no conditions that would prevent valid audiometric testing, such as fluid in the ear, ear infection, or obstruction in an ear canal. The person performing the test should also report on any other factors, such as your ability to maintain attention, that can affect the interpretation of the test results.
c. Children from birth to the attainment of age 6 months.
(i) We need physiologic testing, such as auditory brainstem response (ABR) testing.
(ii) To determine whether your hearing loss meets 102.10A, we will average your hearing thresholds at 500, 1000, 2000, and 4000 Hertz (Hz). If you do not have a response at a particular frequency, we will use a threshold of 5 decibels (dB) over the limit of the audiometer.
d. Children from age 6 months to the attainment of age 2.
(i) We need air conduction thresholds determined by a behavioral assessment, usually visual reinforcement audiometry (VRA). We can use ABR testing if the behavioral assessment cannot be completed or if the results are inconclusive or unreliable.
(ii) To determine whether your hearing loss meets 102.10A, we will average your hearing thresholds at 500, 1000, 2000, and 4000 Hz. If you do not have a response at a particular frequency, we will use a threshold of 5 dB over the limit of the audiometer.
(iii) For this age group, behavioral assessments are often performed in a sound field, and each ear is not tested separately. If each ear is not tested separately, we will consider the test results to represent the hearing in the better ear.
e. Children from age 2 to the attainment of age 5.
(i) We need air conduction thresholds determined by a behavioral assessment, such as conditioned play audiometry (CPA), tangible or visually reinforced operant conditioning audiometry (TROCA, VROCA), or VRA. If you have had ABR testing, we can use the results of that testing if the behavioral assessment cannot be completed or the results are inconclusive or unreliable.
(ii) To determine whether your hearing loss meets 102.10A, we will average your hearing thresholds at 500, 1000, 2000, and 4000 Hz. If you do not have a response at a particular frequency, we will use a threshold of 5 dB over the limit of the audiometer.
(iii) For this age group, behavioral assessments are often performed in a sound field and each ear is not tested separately. If each ear is not tested separately, we will consider the test results to represent the hearing in the better ear.
f. Children from age 5 to the attainment of age 18.
(i) We generally need pure tone air conduction and bone conduction testing, speech reception threshold (SRT) testing (also referred to as “spondee threshold” or “ST” testing), and word recognition testing (also referred to as “word discrimination” or “speech discrimination” testing). This testing must be conducted in a sound-treated booth or room and must be in accordance with the most recently published ANSI standards. Each ear must be tested separately.
(ii) To determine whether your hearing loss meets the air and bone conduction criterion in 102.10B1 or 102.10B3, we will average your hearing thresholds at 500, 1000, 2000, and 4000 Hz. If you do not have a response at a particular frequency, we will use a threshold of 5 dB over the limit of the audiometer.
(iii) The SRT is the minimum dB level required for you to recognize 50 percent of the words on a standard list of spondee words. (Spondee words are two-syllable words that have equal stress on each syllable.) The SRT is usually within 10 dB of the average pure tone air conduction hearing thresholds at 500, 1000, and 2000 Hz. If the SRT is not within 10 dB of the average pure tone air conduction threshold, the reason for the discrepancy must be documented. If we cannot determine that there is a medical basis for the discrepancy, we will not use the results of the testing to determine whether your hearing loss meets a listing.
(iv) Word recognition testing determines your ability to recognize an age-appropriate, standardized list of phonetically balanced monosyllabic words in the absence of any visual cues. This testing must be performed in quiet. The list may be recorded or presented live, but in either case, the words should be presented at a level of amplification that will measure your maximum ability to discriminate words, usually 35 to 40 dB above your SRT. However, the amplification level used in the testing must be medically appropriate, and you must be able to tolerate it. If you cannot be tested at 35 to 40 dB above your SRT, the person who performs the test should report your word recognition testing score at your highest comfortable level of amplification.
g. Screening testing. Physiologic testing, such as ABR and otoacoustic emissions (OAE), and pure tone testing can be used as hearing screening tests. We will not use these tests to determine that your hearing loss meets or medically equals a listing, or to assess functional limitations due to your hearing loss, when they are used only as screening tests. We can consider normal results from hearing screening tests to determine that your hearing loss is not “severe” when these test results are consistent with the other evidence in your case record. See § 416.924(c).
3. What audiometric testing do we need when you have a cochlear implant?
a. If you have a cochlear implant, we will consider you to be disabled until age 5, or for 1 year after initial implantation, whichever is later.
b. After that period, we need word recognition testing performed with any age-appropriate version of the Hearing in Noise Test (HINT) or the Hearing in Noise Test for Children (HINT-C) to determine whether your impairment meets 102.11B. This testing must be conducted in quiet in a sound field. Your implant must be functioning properly and adjusted to your normal settings. The sentences should be presented at 60 dB HL (Hearing Level) and without any visual cues.
4. How do we evaluate your word recognition ability if you are not fluent in English?
If you are not fluent in English, you should have word recognition testing using an appropriate word list for the language in which you are most fluent. The person conducting the test should be fluent in the language used for the test. If there is no appropriate word list or no person who is fluent in the language and qualified to perform the test, it may not be possible to measure your word recognition ability. If your word recognition ability cannot be measured, your hearing loss cannot meet 102.10B2 or 102.11B. Instead, we will consider the facts of your case to determine whether you have difficulty understanding words in the language in which you are most fluent, and if so, whether that degree of difficulty medically equals 102.10B2 or 102.11B. For example, we will consider how you interact with family members, interpreters, and other persons who speak the language in which you are most fluent.
5. What do we mean by a marked limitation in speech or language as used in 102.10B3?
a. We will consider you to have a marked limitation in speech if:
(i) Entire phrases or sentences in your conversation are intelligible to unfamiliar listeners at least 50 percent (half) of the time but no more than 67 percent (two-thirds) of the time on your first attempt; and
(ii) Your sound production or phonological patterns (the ways in which you combine speech sounds) are atypical for your age.
b. We will consider you to have a marked limitation in language when your current and valid test score on an appropriate comprehensive, standardized test of overall language functioning is at least two standard deviations below the mean. In addition, the evidence of your daily communication functioning must be consistent with your test score. If you are not fluent in English, it may not be possible to test your language performance. If we cannot test your language performance, your hearing loss cannot meet 102.10B3. Instead, we will consider the facts of your case to determine whether your hearing loss medically equals 102.10B3.
102.01 Category of Impairments, Special Senses and Speech
102.02 Loss of central visual acuity.
A. Remaining vision in the better eye after best correction is 20/200 or less.
ORB. An inability to participate in visual acuity testing using Snellen methodology or other comparable testing, clinical findings that fixation and visual-following behavior are absent in the better eye, and one of the following:
1. Abnormal anatomical findings indicating a visual acuity of 20/200 or less in the better eye (such as the presence of Stage III or worse retinopathy of prematurity despite surgery, hypoplasia of the optic nerve, albinism with macular aplasia, or bilateral optic atrophy); or
2. Abnormal neuroimaging documenting damage to the cerebral cortex which would be expected to prevent the development of a visual acuity better than 20/200 in the better eye (such as neuroimaging showing bilateral encephalomyelitis or bilateral encephalomalacia); or
3. Abnormal electroretinogram documenting the presence of Leber's congenital amaurosis or achromatopsia in the better eye; or
4. An absent response to VER testing in the better eye.
102.03 Contraction of the visual field in the better eye, with:
A. The widest diameter subtending an angle around the point of fixation no greater than 20 degrees.
ORB. An MD of 22 decibels or greater, determined by automated static threshold perimetry that measures the central 30 degrees of the visual field (see 102.00A6d.).
ORC. A visual field efficiency of 20 percent or less, determined by kinetic perimetry (see 102.00A7c).
102.04 Loss of visual efficiency, or visual impairment, in the better eye:
A. A visual efficiency percentage of 20 or less after best correction (see 102.00A7d.).
ORB. A visual impairment value of 1.00 or greater after best correction (see 102.00A8d).
102.10 Hearing loss not treated with cochlear implantation.
A. For children from birth to the attainment of age 5, an average air conduction hearing threshold of 50 decibels or greater in the better ear (see 102.00B2).
ORB. For children from age 5 to the attainment of age 18:
1. An average air conduction hearing threshold of 70 decibels or greater in the better ear and an average bone conduction hearing threshold of 40 decibels or greater in the better ear (see 102.00B2f); or
2. A word recognition score of 40 percent or less in the better ear determined using a standardized list of phonetically balanced monosyllabic words (see 102.00B2f); or
3. An average air conduction hearing threshold of 50 decibels or greater in the better ear and a marked limitation in speech or language (see 102.00B2f and 102.00B5).
102.11 Hearing loss treated with cochlear implantation.
A. Consider under a disability until the attainment of age 5 or for 1 year after initial implantation, whichever is later.
ORB. Upon the attainment of age 5 or 1 year after initial implantation, whichever is later, a word recognition score of 60 percent or less determined using the HINT or the HINT-C (see 102.00B3b).
103.00 Respiratory DisordersA. Which disorders do we evaluate in this body system?
1. We evaluate respiratory disorders that result in obstruction (difficulty moving air out of the lungs) or restriction (difficulty moving air into the lungs), or that interfere with diffusion (gas exchange) across cell membranes in the lungs. Examples of such disorders and the listings we use to evaluate them include chronic obstructive pulmonary disease (103.02), chronic lung disease of infancy (also known as bronchopulmonary dysplasia, 103.02C or 103.02E), pulmonary fibrosis (103.02), asthma (103.02 or 103.03), and cystic fibrosis (103.04). We also use listings in this body system to evaluate respiratory failure resulting from an underlying chronic respiratory disorder (103.04E or 103.14) and lung transplantation (103.11).
2. We evaluate cancers affecting the respiratory system under the listings in 113.00. We evaluate the pulmonary effects of neuromuscular and autoimmune disorders under these listings or under the listings in 111.00 or 114.00, respectively.
B. What are the symptoms and signs of respiratory disorders? Symptoms and signs of respiratory disorders include dyspnea (shortness of breath), chest pain, coughing, wheezing, sputum production, hemoptysis (coughing up blood from the respiratory tract), use of accessory muscles of respiration, and tachypnea (rapid rate of breathing).
C. What abbreviations do we use in this body system?
1. BiPAP means bi-level positive airway pressure ventilation.
2. BTPS means body temperature and ambient pressure, saturated with water vapor.
3. CF means cystic fibrosis.
4. CFRD means CF-related diabetes.
5. CFTR means CF transmembrane conductance regulator.
6. CLD means chronic lung disease of infancy.
7. FEV1 means forced expiratory volume in the first second of a forced expiratory maneuver.
8. FVC means forced vital capacity.
9. L means liter.
D. What documentation do we need to evaluate your respiratory disorder?
1. We need medical evidence to document and assess the severity of your respiratory disorder. Medical evidence should include your medical history, physical examination findings, the results of imaging (see 103.00D3), spirometry (see 103.00E), other relevant laboratory tests, and descriptions of any prescribed treatment and your response to it. We may not need all of this evidence depending on your particular respiratory disorder and its effects on you.
2. If you use supplemental oxygen, we still need medical evidence to establish the severity of your respiratory disorder.
3. Imaging refers to medical imaging techniques, such as x-ray and computerized tomography. The imaging must be consistent with the prevailing state of medical knowledge and clinical practice as the proper technique to support the evaluation of the disorder.
E. What is spirometry and what are our requirements for an acceptable test and report?
1. Spirometry, which measures how well you move air into and out of your lungs, involves at least three forced expiratory maneuvers during the same test session. A forced expiratory maneuver is a maximum inhalation followed by a forced maximum exhalation, and measures exhaled volumes of air over time. The volume of air you exhale in the first second of the forced expiratory maneuver is the FEV1. The total volume of air that you exhale during the entire forced expiratory maneuver is the FVC. We use your highest FEV1 value to evaluate your respiratory disorder under 103.02A and 103.04A, and your highest FVC value to evaluate your respiratory disorder under 103.02B, regardless of whether the values are from the same forced expiratory maneuver or different forced expiratory maneuvers. We will not purchase spirometry for children who have not attained age 6.
2. We have the following requirements for spirometry under these listings:
a. You must be medically stable at the time of the test. Examples of when we would not consider you to be medically stable include when you are:
(i) Within 2 weeks of a change in your prescribed respiratory medication.
(ii) Experiencing, or within 30 days of completion of treatment for, a lower respiratory tract infection.
(iii) Experiencing, or within 30 days of completion of treatment for, an acute exacerbation (temporary worsening) of a chronic respiratory disorder. Wheezing by itself does not indicate that you are not medically stable.
b. During testing, if your FEV1 is less than 70 percent of your predicted normal value, we require repeat spirometry after inhalation of a bronchodilator to evaluate your respiratory disorder under these listings, unless it is medically contraindicated. If you used a bronchodilator before the test and your FEV1 is less than 70 percent of your predicted normal value, we still require repeat spirometry after inhalation of a bronchodilator unless the supervising physician determines that it is not safe for you to take a bronchodilator again (in which case we may need to reschedule the test). If you do not have post-bronchodilator spirometry, the test report must explain why. We can use the results of spirometry administered without bronchodilators when the use of bronchodilators is medically contraindicated.
c. Your forced expiratory maneuvers must be satisfactory. We consider a forced expiratory maneuver to be satisfactory when you exhale with maximum effort following a full inspiration, and when the test tracing has a sharp takeoff and rapid rise to peak flow, has a smooth contour, and either lasts for at least 6 seconds (for children age 10 and older) or for at least 3 seconds (for children who have not attained age 10), or maintains a plateau for at least 1 second.
3. The spirometry report must include the following information:
a. The date of the test and your name, age or date of birth, gender, and height without shoes. (We will assume that your recorded height on the date of the test is without shoes, unless we have evidence to the contrary.) If your spine is abnormally curved (for example, you have kyphoscoliosis), we will substitute the longest distance between your outstretched fingertips with your arms abducted 90 degrees in place of your height when this measurement is greater than your standing height without shoes.
b. Any factors, if applicable, that can affect the interpretation of the test results (for example, your cooperation or effort in doing the test).
c. Legible tracings of your forced expiratory maneuvers in a volume-time format showing your name and the date of the test for each maneuver.
4. If you have attained age 6, we may need to purchase spirometry to determine whether your disorder meets a listing, unless we can make a fully favorable determination or decision on another basis.
5. Before we purchase spirometry for a child age 6 or older, a medical consultant (see § 416.1016 of this chapter), preferably one with experience in the care of children with respiratory disorders, must review your case record to determine if we need the test. If we purchase spirometry, the medical source we designate to administer the test is solely responsible for deciding whether it is safe for you to do the test and for how to administer it.
F. What is CLD and how do we evaluate it?
1. CLD, also known as bronchopulmonary dysplasia, or BPD, is scarring of the immature lung. CLD may develop as a complication of mechanical ventilation and oxygen therapy for infants with significant neonatal respiratory problems. Within the first 6 months of life, most infants with CLD are successfully weaned from mechanical ventilation, and then weaned from oxygen supplementation. We evaluate CLD under 103.02C, 103.02E, or if you are age 2 or older, under 103.03 or another appropriate listing.
2. If you have CLD, are not yet 6 months old, and need 24-hour-per-day oxygen supplementation, we will not evaluate your CLD under 103.02C until you are 6 months old. Depending on the evidence in your case record, we may make a fully favorable determination or decision under other rules before you are 6 months old.
3. We evaluate your CLD under 103.02C if you are at least 6 months old and you need 24-hour-per-day oxygen supplementation. (If you were born prematurely, we use your corrected chronological age. See § 416.924b(b) of this chapter.) We also evaluate your CLD under 103.02C if you were weaned off oxygen supplementation but needed it again by the time you were 6 months old or older.
4. We evaluate your CLD under 103.02E if you are any age from birth to the attainment of age 2 and have CLD exacerbations or complications (for example, wheezing, lower respiratory tract infections, or acute respiratory distress) that require hospitalization. For the purpose of 103.02E, we count your initial birth hospitalization as one hospitalization. The phrase “consider under a disability for 1 year from the discharge date of the last hospitalization or until the attainment of age 2, whichever is later” in 103.02E does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your impairment(s) continues to meet a listing or is otherwise disabling.
G. What is asthma and how do we evaluate it?
1. Asthma is a chronic inflammatory disorder of the lung airways that we evaluate under 103.02 or 103.03. If you have respiratory failure resulting from chronic asthma (see 103.00J), we will evaluate it under 103.14.
2. For the purposes of 103.03:
a. The phrase “consider under a disability for 1 year” explains how long your asthma can meet the requirements of the listing. It does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your asthma continues to meet a listing or is otherwise disabling.
b. We determine the onset of your disability based on the facts of your case, but it will be no later than the admission date of your first of three hospitalizations that satisfy the criteria of 103.03.
H. What is CF and how do we evaluate it?
1. General. We evaluate CF, a genetic disorder that results in abnormal salt and water transport across cell membranes in the lungs, pancreas, and other body organs, under 103.04. We need the evidence described in 103.00H2 to establish that you have CF.
2. Documentation of CF. We need a report signed by a physician (see § 416.913(a) of this chapter) showing both a and b:
a. One of the following:
(i) A positive newborn screen for CF; or
(ii) A history of CF in a sibling; or
(iii) Documentation of at least one specific CF phenotype or clinical criterion (for example, chronic sino-pulmonary disease with persistent colonization or infections with typical CF pathogens, pancreatic insufficiency, or salt-loss syndromes); and
b. One of the following definitive laboratory tests:
(i) An elevated sweat chloride concentration equal to or greater than 60 millimoles per L; or
(ii) The identification of two CF gene mutations affecting the CFTR; or
(iii) Characteristic abnormalities in ion transport across the nasal epithelium.
c. When we have the report showing a and b, but it is not signed by a physician, we also need a report from a physician stating that you have CF.
d. When we do not have the report showing a and b, we need a report from a physician that is persuasive that a positive diagnosis of CF was confirmed by an appropriate definitive laboratory test. To be persuasive, this report must include a statement by the physician that you had the appropriate definitive laboratory test for diagnosing CF. The report must provide the test results or explain how your diagnosis was established that is consistent with the prevailing state of medical knowledge and clinical practice.
3. CF pulmonary exacerbations. Examples of CF pulmonary exacerbations include increased cough and sputum production, hemoptysis, increased shortness of breath, increased fatigue, and reduction in pulmonary function. Treatment usually includes intravenous antibiotics and intensified airway clearance therapy (for example, increased frequencies of chest percussion or increased use of inhaled nebulized therapies, such as bronchodilators or mucolytics).
4. For 103.04G, we require any two exacerbations or complications from the list in 103.04G1 through 103.04G4 within a 12-month period. You may have two of the same exacerbation or complication or two different ones.
a. If you have two of the acute exacerbations or complications we describe in 103.04G1 and 103.04G2, there must be at least 30 days between the two.
b. If you have one of the acute exacerbations or complications we describe in 103.04G1 and 103.04G2 and one of the chronic complications we describe in 103.04G3 and 103.04G4, the two can occur during the same time. For example, your CF meets 103.04G if you have the pulmonary hemorrhage we describe in 103.04G2 and the weight loss we describe in 103.04G3 even if the pulmonary hemorrhage occurs during the 90-day period in 103.04G3.
c. Your CF also meets 103.04G if you have both of the chronic complications in 103.04G3 and 103.04G4.
5. CF may also affect other body systems such as digestive or endocrine. If your CF, including pulmonary exacerbations and nonpulmonary complications, does not meet or medically equal a respiratory disorders listing, we may evaluate your CF-related impairments under the listings in the affected body system.
I. How do we evaluate lung transplantation? If you receive a lung transplant (or a lung transplant simultaneously with other organs, such as the heart), we will consider you to be disabled under 103.11 for 3 years from the date of the transplant. After that, we evaluate your residual impairment(s) by considering the adequacy of your post-transplant function, the frequency and severity of any rejection episodes you have, complications in other body systems, and adverse treatment effects. Children who receive organ transplants generally have impairments that meet our definition of disability before they undergo transplantation. The phrase “consider under a disability for 3 years” in 103.11 does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your impairment(s) continues to meet a listing or is otherwise disabling. We determine the onset of your disability based on the facts of your case.
J. What is respiratory failure and how do we evaluate it? Respiratory failure is the inability of the lungs to perform their basic function of gas exchange. We evaluate respiratory failure under 103.04E if you have CF-related respiratory failure, or under 103.14 if you have respiratory failure due to any other chronic respiratory disorder. Continuous positive airway pressure does not satisfy the criterion in 103.04E or 103.14, and cannot be substituted as an equivalent finding, for invasive mechanical ventilation or noninvasive ventilation with BiPAP.
K. How do we evaluate growth failure due to any chronic respiratory disorder?
1. To evaluate growth failure due to any chronic respiratory disorder, we require documentation of the oxygen supplementation described in 103.06A and the growth measurements in 103.06B within the same consecutive 12-month period. The dates of oxygen supplementation may be different from the dates of growth measurements.
2. Under 103.06B, we use the appropriate table(s) under 105.08B in the digestive system to determine whether a child's growth is less than the third percentile.
a. For children from birth to attainment of age 2, we use the weight-for-length table corresponding to the child's gender (Table I or Table II).
b. For children age 2 to attainment of age 18, we use the body mass index (BMI)-for-age table corresponding to the child's gender (Table III or Table IV).
c. BMI is the ratio of a child's weight to the square of his or her height. We calculate BMI using the formulas in 105.00G2c.
L. How do we evaluate respiratory disorders that do not meet one of these listings?
1. These listings are only examples of common respiratory disorders that we consider severe enough to result in marked and severe functional limitations. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that meets the criteria of a listing in another body system. For example, if your CF has resulted in chronic pancreatic or hepatobiliary disease, we evaluate your impairment under the listings in 105.00.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. See § 416.926 of this chapter. Respiratory disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not meet or medically equal a listing, we will also consider whether it functionally equals the listings. See § 416.926a of this chapter. We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
103.01 Category of Impairments, Respiratory Disorders103.02 Chronic respiratory disorders due to any cause except CF (for CF, see 103.04), with A, B, C, D, or E:
A. FEV1 (see 103.00E) less than or equal to the value in Table I-A or I-B for your age, gender, and height without shoes (see 103.00E3a).
Table I - FEV1 Criteria for 103.02A
| Table I-A | Table I-B | |||||
|---|---|---|---|---|---|---|
| Age 6 to
attainment of age 13 (for both females and males) |
Age 13 to attainment of age 18 | |||||
| Height without shoes (centimeters) < means less than |
Height without shoes (inches) < means less than |
FEV1 less than or equal to (L, BTPS) |
Height without shoes (centimeters) < means less than |
Height without shoes (inches) < means less than |
Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
| <123.0 | <48.50 | 0.80 | <153.0 | <60.25 | 1.35 | 1.40 |
| 123.0 to <129.0 | 48.50 to <50.75 | 0.90 | 153.0 to <159.0 | 60.25 to <62.50 | 1.45 | 1.50 |
| 129.0 to <134.0 | 50.75 to <52.75 | 1.00 | 159.0 to <164.0 | 62.50 to <64.50 | 1.55 | 1.60 |
| 134.0 to <139.0 | 52.75 to <54.75 | 1.10 | 164.0 to <169.0 | 64.50 to <66.50 | 1.65 | 1.70 |
| 139.0 to <144.0 | 54.75 to <56.75 | 1.20 | 169.0 to <174.0 | 66.50 to <68.50 | 1.75 | 1.85 |
| 144.0 to <149.0 | 56.75 to <58.75 | 1.30 | 174.0 to <180.0 | 68.50 to <70.75 | 1.85 | 2.00 |
| 149.0 or more | 58.75 or more | 1.40 | 180.0 or more | 70.75 or more | 1.95 | 2.10 |
OR
B. FVC (see 103.00E) less than or equal to the value in Table II-A or II-B for your age, gender, and height without shoes (see 103.00E3a).
Table II - FVC Criteria for 103.02B
| Table II-A | Table II-B | |||||
|---|---|---|---|---|---|---|
| Age 6 to
attainment of age 13 (for both females and males) |
Age 13 to attainment of age 18 | |||||
| Height without shoes
(centimeters) < means less than |
Height without shoes (inches) < means less than |
FVC less than or equal to (L, BTPS) |
Height without shoes (centimeters) < means less than |
Height without shoes (inches) < means less than |
Females FVC less than or equal to (L, BTPS) |
Males FVC less than or equal to (L, BTPS) |
| <123.0 | <48.50 | 0.85 | <153.0 | <60.25 | 1.65 | 1.65 |
| 123.0 to <129.0 | 48.50 to <50.75 | 1.00 | 153.0 to <159.0 | 60.25 to <62.50 | 1.70 | 1.80 |
| 129.0 to <134.0 | 50.75 to <52.75 | 1.10 | 159.0 to <164.0 | 62.50 to <64.50 | 1.80 | 1.95 |
| 134.0 to <139.0 | 52.75 to <54.75 | 1.30 | 164.0 to <169.0 | 64.50 to <66.50 | 1.95 | 2.10 |
| 139.0 to <144.0 | 54.75 to <56.75 | 1.40 | 169.0 to <174.0 | 66.50 to <68.50 | 2.05 | 2.25 |
| 144.0 to <149.0 | 56.75 to <58.75 | 1.55 | 174.0 to <180.0 | 68.50 to <70.75 | 2.20 | 2.45 |
| 149.0 or more | 58.75 or more | 1.70 | 180.0 or more | 70.75 or more | 2.30 | 2.55 |
OR
C. Hypoxemia with the need for at least 1.0 L per minute of continuous (24 hours per day) oxygen supplementation for at least 90 consecutive days.
OR
D. The presence of a tracheostomy.
1. Consider under a disability until the attainment of age 3; or
2. Upon the attainment of age 3, documented need for mechanical ventilation via a tracheostomy for at least 4 hours per day and for at least 90 consecutive days.
OR
E. For children who have not attained age 2, CLD (see 103.00F) with exacerbations or complications requiring three hospitalizations within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization. (A child's initial birth hospitalization when CLD is first diagnosed counts as one hospitalization.) Consider under a disability for 1 year from the discharge date of the last hospitalization or until the attainment of age 2, whichever is later. After that, evaluate the impairment(s) under 103.03 or another appropriate listing.
103.03 Asthma (see 103.00G) with exacerbations or complications requiring three hospitalizations within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization. Consider under a disability for 1 year from the discharge date of the last hospitalization; after that, evaluate the residual impairment(s) under 103.03 or another appropriate listing.
103.04 Cystic fibrosis (documented as described in 103.00H), with A, B, C, D, E, F, or G:
A. FEV1 (see 103.00E) less than or equal to the value in Table III-A or Table III-B for your age, gender, and height without shoes (see 103.00E3a).
Table III - FEV1 Criteria for 103.04A
| Table III-A | Table III-B | |||||
|---|---|---|---|---|---|---|
| Age 6 to
attainment of age 13 (for both females and males) |
Age 13 to attainment of age 18 | |||||
| Height without shoes
(centimeters) < means less than |
Height without shoes (inches) < means less than |
FEV1 less than or equal to (L, BTPS) |
Height without shoes
(centimeters) < means less than |
Height without shoes (inches) < means less than |
Females FEV1 less than or equal to (L, BTPS) |
Males FEV1 less than or equal to (L, BTPS) |
| <123.0 | <48.50 | 1.00 | <153.0 | <60.25 | 1.75 | 1.85 |
| 123.0 to <129.0 | 48.50 to <50.75 | 1.15 | 153.0 to <159.0 | 60.25 to <62.50 | 1.85 | 2.05 |
| 129.0 to <134.0 | 50.75 to <52.75 | 1.25 | 159.0 to <164.0 | 62.50 to <64.50 | 1.95 | 2.15 |
| 134.0 to <139.0 | 52.75 to <54.75 | 1.40 | 164.0 to <169.0 | 64.50 to <66.50 | 2.10 | 2.30 |
| 139.0 to <144.0 | 54.75 to <56.75 | 1.50 | 169.0 to <174.0 | 66.50 to <68.50 | 2.25 | 2.45 |
| 144.0 to <149.0 | 56.75 to <58.75 | 1.70 | 174.0 to <180.0 | 68.50 to <70.75 | 2.35 | 2.60 |
| 149.0 or more | 58.75 or more | 1.80 | 180.0 or more | 70.75 or more | 2.50 | 2.70 |
OR
B. For children who have not attained age 6, findings on imaging (see 103.00D3) of thickening of the proximal bronchial airways, nodular-cystic lesions, segmental or lobular atelectasis, or consolidation, and documentation of one of the following:
1. Shortness of breath with activity; or
2. Accumulation of secretions as manifested by repetitive coughing; or
3. Bilateral rales or rhonchi, or reduction of breath sounds.
OR
C. Exacerbations or complications (see 103.00H3) requiring three hospitalizations of any length within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review).
OR
D. Spontaneous pneumothorax, secondary to CF, requiring chest tube placement.
OR
E. Respiratory failure (see 103.00J) requiring invasive mechanical ventilation, noninvasive ventilation with BiPAP, or a combination of both treatments, for a continuous period of at least 48 hours, or for a continuous period of at least 72 hours if postoperatively.
OR
F. Pulmonary hemorrhage requiring vascular embolization to control bleeding.
OR
G. Two of the following exacerbations or complications (either two of the same or two different, see 103.00H3 and 103.00H4) within a 12-month period (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review):
1. Pulmonary exacerbation requiring 10 consecutive days of intravenous antibiotic treatment.
2. Pulmonary hemorrhage (hemoptysis with more than blood-streaked sputum but not requiring vascular embolization) requiring hospitalization of any length.
3. Weight loss requiring daily supplemental enteral nutrition via a gastrostomy for at least 90 consecutive days or parenteral nutrition via a central venous catheter for at least 90 consecutive days.
4. CFRD requiring daily insulin therapy for at least 90 consecutive days.
103.05 [Reserved]
103.06 Growth failure due to any chronic respiratory disorder (see 103.00K), documented by:
A. Hypoxemia with the need for at least 1.0 L per min of oxygen supplementation for at least 4 hours per day and for at least 90 consecutive days.
AND
B. Growth failure as required in 1 or 2:
1. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate weight-for-length table under 105.08B1; or
2. For children age 2 to attainment of age 18, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate BMI-for-age table under 105.08B2.
103.07 [Reserved]
103.08 [Reserved]
103.09 [Reserved]
103.10 [Reserved]
103.11 Lung transplantation (see 103.00I). Consider under a disability for 3 years from the date of the transplant; after that, evaluate the residual impairment(s).
103.12 [Reserved]
103.13 [Reserved]
103.14 Respiratory failure (see 103.00J) resulting from any underlying chronic respiratory disorder except CF (for CF, see 103.04E), requiring invasive mechanical ventilation, noninvasive ventilation with BiPAP, or a combination of both treatments, for a continuous period of at least 48 hours, or for a continuous period of at least 72 hours if postoperatively, twice within a 12-month period and at least 30 days apart (the 12-month period must occur within the period we are considering in connection with your application or continuing disability review).
104.00 Cardiovascular System A. General1. What do we mean by a cardiovascular impairment?
a. We mean any disorder that affects the proper functioning of the heart or the circulatory system (that is, arteries, veins, capillaries, and the lymphatic drainage). The disorder can be congenital or acquired.
b. Cardiovascular impairment results from one or more of four consequences of heart disease:
(i) Chronic heart failure or ventricular dysfunction.
(ii) Discomfort or pain due to myocardial ischemia, with or without necrosis of heart muscle.
(iii) Syncope, or near syncope, due to inadequate cerebral perfusion from any cardiac cause, such as obstruction of flow or disturbance in rhythm or conduction resulting in inadequate cardiac output.
(iv) Central cyanosis due to right-to-left shunt, reduced oxygen concentration in the arterial blood, or pulmonary vascular disease.
c. Disorders of the veins or arteries (for example, obstruction, rupture, or aneurysm) may cause impairments of the lower extremities (peripheral vascular disease), the central nervous system, the eyes, the kidneys, and other organs. We will evaluate peripheral vascular disease under 4.11 or 4.12 in part A, and impairments of another body system(s) under the listings for that body system(s).
2. What do we consider in evaluating cardiovascular impairments? The listings in this section describe cardiovascular impairments based on symptoms, signs, laboratory findings, response to a regimen of prescribed treatment, and functional limitations.
3. What do the following terms or phrases mean in these listings?
a. Medical consultant is an individual defined in §§ 404.1616(a) and 416.1016(a). This term does not include medical sources who provide consultative examinations for us. We use the abbreviation “MC” throughout this section to designate a medical consultant.
b. Persistent means that the longitudinal clinical record shows that, with few exceptions, the required finding(s) has been present, or is expected to be present, for a continuous period of at least 12 months, such that a pattern of continuing severity is established.
c. Recurrent means that the longitudinal clinical record shows that, within a consecutive 12-month period, the finding(s) occurs at least three times, with intervening periods of improvement of sufficient duration that it is clear that separate events are involved.
d. Appropriate medically acceptable imaging means that the technique used is the proper one to evaluate and diagnose the impairment and is commonly recognized as accurate for assessing the cited finding.
e. A consecutive 12-month period means a period of 12 consecutive months, all or part of which must occur within the period we are considering in connection with an application or continuing disability review.
f. Currently present means that the finding is present at the time of adjudication.
g. Uncontrolled means the impairment does not respond adequately to standard prescribed medical treatment.
B. Documenting Cardiovascular Impairment1. What basic documentation do we need? We need sufficiently detailed reports of history, physical examinations, laboratory studies, and any prescribed treatment and response to allow us to assess the severity and duration of your cardiovascular impairment. A longitudinal clinical record covering a period of not less than 3 months of observations and treatment is usually necessary, unless we can make a determination or decision based on the current evidence.
2. Why is a longitudinal clinical record important? We will usually need a longitudinal clinical record to assess the severity and expected duration of your impairment(s). If you have a listing-level impairment, you probably will have received medically prescribed treatment. Whenever there is evidence of such treatment, your longitudinal clinical record should include a description of the ongoing management and evaluation provided by your treating or other medical source. It should also include your response to this medical management, as well as information about the nature and severity of your impairment. The record will provide us with information on your functional status over an extended period of time and show whether your ability to function is improving, worsening, or unchanging.
3. What if you have not received ongoing medical treatment?
a. You may not have received ongoing treatment or have an ongoing relationship with the medical community despite the existence of a severe impairment(s). In this situation, we will base our evaluation on the current objective medical evidence and the other evidence we have. If you do not receive treatment, you cannot show an impairment that meets the criteria of these listings. However, we may find you disabled because you have another impairment(s) that in combination with your cardiovascular impairment medically equals the severity of a listed impairment or that functionally equals the listings.
b. Unless we can decide your claim favorably on the basis of the current evidence, a longitudinal record is still important. In rare instances where there is no or insufficient longitudinal evidence, we may purchase a consultative examination(s) to help us establish the severity and duration of your impairment.
4. When will we wait before we ask for more evidence?
a. We will wait when we have information showing that your impairment is not yet stable and the expected change in your impairment might affect our determination or decision. In these situations, we need to wait to properly evaluate the severity and duration of your impairment during a stable period. Examples of when we might wait are:
(i) If you have had a recent acute event; for example, acute rheumatic fever.
(ii) If you have recently had a corrective cardiac procedure; for example, open-heart surgery.
(iii) If you have started new drug therapy and your response to this treatment has not yet been established; for example, beta-blocker therapy for dilated congestive cardiomyopathy.
b. In these situations, we will obtain more evidence 3 months following the event before we evaluate your impairment. However, we will not wait if we have enough information to make a determination or decision based on all of the relevant evidence in your case.
5. Will we purchase any studies? In appropriate situations, we will purchase studies necessary to substantiate the diagnosis or to document the severity of your impairment, generally after we have evaluated the medical and other evidence we already have. We will not purchase studies involving exercise testing if there is significant risk involved or if there is another medical reason not to perform the test. We will follow sections 4.00C6, 4.00C7, 4.00C8, and 104.00B7 when we decide whether to purchase exercise testing. We will make a reasonable effort to obtain any additional studies from a qualified medical source in an office or center experienced in pediatric cardiac assessment. (See § 416.919g.)
6. What studies will we not purchase? We will not purchase any studies involving cardiac catheterization, such as coronary angiography, arteriograms, or electrophysiological studies. However, if the results of catheterization are part of the existing evidence we have, we will consider them together with the other relevant evidence. See 4.00C15a in part A.
7. Will we use exercise tolerance tests (ETTs) for evaluating children with cardiovascular impairment?
a. ETTs, though increasingly used, are still less frequently indicated in children than in adults, and can rarely be performed successfully by children under 6 years of age. An ETT may be of value in the assessment of some arrhythmias, in the assessment of the severity of chronic heart failure, and in the assessment of recovery of function following cardiac surgery or other treatment.
b. We will purchase an ETT in a childhood claim only if we cannot make a determination or decision based on the evidence we have and an MC, preferably one with experience in the care of children with cardiovascular impairments, has determined that an ETT is needed to evaluate your impairment. We will not purchase an ETT if you are less than 6 years of age. If we do purchase an ETT for a child age 12 or younger, it must be performed by a qualified medical source in a specialty center for pediatric cardiology or other facility qualified to perform exercise tests of children.
c. For full details on ETT requirements and usage, see 4.00C in part A.
C. Evaluating Chronic Heart Failure1. What is chronic heart failure (CHF)?
a. CHF is the inability of the heart to pump enough oxygenated blood to body tissues. This syndrome is characterized by symptoms and signs of pulmonary or systemic congestion (fluid retention) or limited cardiac output. Certain laboratory findings of cardiac functional and structural abnormality support the diagnosis of CHF.
b. CHF is considered in these listings as a single category whether due to atherosclerosis (narrowing of the arteries), cardiomyopathy, hypertension, or rheumatic, congenital, or other heart disease. However, if the CHF is the result of primary pulmonary hypertension secondary to disease of the lung (cor pulmonale), we will evaluate your impairment using 3.09 in the respiratory system listings in part A.
2. What evidence of CHF do we need?
a. Cardiomegaly or ventricular dysfunction must be present and demonstrated by appropriate medically acceptable imaging, such as chest x-ray, echocardiography (M-Mode, 2-dimensional, and Doppler), radionuclide studies, or cardiac catheterization.
(i) Cardiomegaly is present when:
(A) Left ventricular diastolic dimension or systolic dimension is greater than 2 standard deviations above the mean for the child's body surface area;
(B) Left ventricular mass is greater than 2 standard deviations above the mean for the child's body surface area; or
(C) Chest x-ray (6 foot PA film) is indicative of cardiomegaly if the cardiothoracic ratio is over 60 percent at 1 year of age or less, or 55 percent or greater at more than 1 year of age.
(ii) Ventricular dysfunction is present when indices of left ventricular function, such as fractional shortening or ejection fraction (the percentage of the blood in the ventricle actually pumped out with each contraction), are greater than 2 standard deviations below the mean for the child's age. (Fractional shortening, also called shortening fraction, reflects the left ventricular systolic function in the absence of segmental wall motion abnormalities and has a linear correlation with ejection fraction. In children, fractional shortening is more commonly used than ejection fraction.)
(iii) However, these measurements alone do not reflect your functional capacity, which we evaluate by considering all of the relevant evidence.
(iv) Other findings on appropriate medically acceptable imaging may include increased pulmonary vascular markings, pleural effusion, and pulmonary edema. These findings need not be present on each report, since CHF may be controlled by prescribed treatment.
b. To establish that you have chronic heart failure, we require that your medical history and physical examination describe characteristic symptoms and signs of pulmonary or systemic congestion or of limited cardiac output associated with abnormal findings on appropriate medically acceptable imaging. When a remediable factor, such as arrhythmia, triggers an acute episode of heart failure, you may experience restored cardiac function, and a chronic impairment may not be present.
(i) Symptoms of congestion or of limited cardiac output include easy fatigue, weakness, shortness of breath (dyspnea), cough, or chest discomfort at rest or with activity. Children with CHF may also experience shortness of breath on lying flat (orthopnea) or episodes of shortness of breath that wake them from sleep (paroxysmal nocturnal dyspnea). They may also experience cardiac arrhythmias resulting in palpitations, lightheadedness, or fainting. Fatigue or exercise intolerance in an infant may be manifested by prolonged feeding time, often associated with excessive respiratory effort and sweating.
(ii) During infancy, other manifestations of chronic heart failure may include repeated lower respiratory tract infections.
(iii) Signs of congestion may include hepatomegaly, ascites, increased jugular venous distention or pressure, rales, peripheral edema, rapid shallow breathing (tachypnea), or rapid weight gain. However, these signs need not be found on all examinations because fluid retention may be controlled by prescribed treatment.
3. How do we evaluate growth failure due to CHF?
a. To evaluate growth failure due to CHF, we require documentation of the clinical findings of CHF described in 104.00C2 and the growth measurements in 104.02C within the same consecutive 12-month period. The dates of clinical findings may be different from the dates of growth measurements.
b. Under 104.02C, we use the appropriate table(s) under 105.08B in the digestive system to determine whether a child's growth is less than the third percentile.
(i) For children from birth to attainment of age 2, we use the weight-for-length table corresponding to the child's gender (Table I or Table II).
(ii) For children age 2 to attainment of age 18, we use the body mass index (BMI)-for-age table corresponding to the child's gender (Table III or Table IV).
(iii) BMI is the ratio of a child's weight to the square of his or her height. We calculate BMI using the formulas in 105.00G2c.
D. Evaluating Congenital Heart Disease1. What is congenital heart disease? Congenital heart disease is any abnormality of the heart or the major blood vessels that is present at birth. Examples include:
a. Abnormalities of cardiac septation, including ventricular septal defect or atrioventricular canal;
b. Abnormalities resulting in cyanotic heart disease, including tetralogy of Fallot or transposition of the great arteries;
c. Valvular defects or obstructions to ventricular outflow, including pulmonary or aortic stenosis or coarctation of the aorta; and
d. Major abnormalities of ventricular development, including hypoplastic left heart syndrome or pulmonary tricuspid atresia with hypoplastic right ventricle.
2. How will we evaluate symptomatic congenital heart disease?
a. Because of improved treatment methods, more children with congenital heart disease are living longer. Although some types of congenital heart disease may be corrected by surgery, many children with treated congenital heart disease continue to have problems throughout their lives (symptomatic congenital heart disease). If you have congenital heart disease that results in chronic heart failure with evidence of ventricular dysfunction or in recurrent arrhythmias, we will evaluate your impairment under 104.02 or 104.05. Otherwise, we will evaluate your impairment under 104.06.
b. For 104.06A2, we will accept pulse oximetry measurements instead of arterial O2, but the arterial O2 values are preferred, if available.
c. For 104.06D, examples of impairments that in most instances will require life-saving surgery or a combination of surgery and other major interventional procedures (for example, multiple “balloon” catheter procedures) before age 1 include, but are not limited to, the following:
(i) Hypoplastic left heart syndrome,
(ii) Critical aortic stenosis with neonatal heart failure,
(iii) Critical coarctation of the aorta, with or without associated anomalies,
(iv) Complete atrioventricular canal defects,
(v) Transposition of the great arteries,
(vi) Tetralogy of Fallot,
(vii) Pulmonary atresia with intact ventricular septum,
(viii) Single ventricle,
(ix) Tricuspid atresia, and
(x) Multiple ventricular septal defects.
E. Evaluating Arrhythmias1. What is an arrhythmia? An arrhythmia is a change in the regular beat of the heart. Your heart may seem to skip a beat or beat irregularly, very quickly (tachycardia), or very slowly (bradycardia).
2. What are the different types of arrhythmias?
a. There are many types of arrhythmias. Arrhythmias are identified by where they occur in the heart (atria or ventricles) and by what happens to the heart's rhythm when they occur.
b. Arrhythmias arising in the cardiac atria (upper chambers of the heart) are called atrial or supraventricular arrhythmias. Ventricular arrhythmias begin in the ventricles (lower chambers). In general, ventricular arrhythmias caused by heart disease are the most serious.
3. How do we evaluate arrhythmias using 104.05?
a. We will use 104.05 when you have arrhythmias that are not fully controlled by medication, an implanted pacemaker, or an implanted cardiac defibrillator and you have uncontrolled recurrent episodes of syncope or near syncope. If your arrhythmias are controlled, we will evaluate your underlying heart disease using the appropriate listing. For other considerations when we evaluate arrhythmias in the presence of an implanted cardiac defibrillator, see 104.00E4.
b. We consider near syncope to be a period of altered consciousness, since syncope is a loss of consciousness or a faint. It is not merely a feeling of light-headedness, momentary weakness, or dizziness.
c. For purposes of 104.05, there must be a documented association between the syncope or near syncope and the recurrent arrhythmia. The recurrent arrhythmia, not some other cardiac or non-cardiac disorder, must be established as the cause of the associated symptom. This documentation of the association between the symptoms and the arrhythmia may come from the usual diagnostic methods, including Holter monitoring (also called ambulatory electrocardiography) and tilt-table testing with a concurrent ECG. Although an arrhythmia may be a coincidental finding on an ETT, we will not purchase an ETT to document the presence of a cardiac arrhythmia.
4. What will we consider when you have an implanted cardiac defibrillator and you do not have arrhythmias that meet the requirements of 104.05?
a. Implanted cardiac defibrillators are used to prevent sudden cardiac death in children who have had, or are at high risk for, cardiac arrest from life-threatening ventricular arrhythmias. The largest group of children at risk for sudden cardiac death consists of children with cardiomyopathy (ischemic or non-ischemic) and reduced ventricular function. However, life-threatening ventricular arrhythmias can also occur in children with little or no ventricular dysfunction. The shock from the implanted cardiac defibrillator is a unique form of treatment; it rescues a child from what may have been cardiac arrest. However, as a consequence of the shock(s), children may experience psychological distress, which we may evaluate under the mental disorders listings in 112.00ff.
b. Most implantable cardiac defibrillators have rhythm-correcting and pacemaker capabilities. In some children, these functions may result in the termination of ventricular arrhythmias without an otherwise painful shock. (The shock is like being kicked in the chest.) Implanted cardiac defibrillators may deliver inappropriate shocks, often repeatedly, in response to benign arrhythmias or electrical malfunction. Also, exposure to strong electrical or magnetic fields, such as from MRI (magnetic resonance imaging), can trigger or reprogram an implanted cardiac defibrillator, resulting in inappropriate shocks. We must consider the frequency of, and the reason(s) for, the shocks when evaluating the severity and duration of your impairment.
c. In general, the exercise limitations imposed on children with an implanted cardiac defibrillator are those dictated by the underlying heart impairment. However, the exercise limitations may be greater when the implanted cardiac defibrillator delivers an inappropriate shock in response to the increase in heart rate with exercise, or when there is exercise-induced ventricular arrhythmia.
F. Evaluating Other Cardiovascular Impairments1. What is ischemic heart disease (IHD) and how will we evaluate it in children? IHD results when one or more of your coronary arteries is narrowed or obstructed or, in rare situations, constricted due to vasospasm, interfering with the normal flow of blood to your heart muscle (ischemia). The obstruction may be the result of an embolus, a thrombus, or plaque. When heart muscle tissue dies as a result of the reduced blood supply, it is called a myocardial infarction (heart attack). Ischemia is rare in children, but when it occurs, its effects on children are the same as on adults. If you have IHD, we will evaluate it under 4.00E and 4.04 in part A.
2. How will we evaluate hypertension? Because hypertension (high blood pressure) generally causes disability through its effects on other body systems, we will evaluate it by reference to the specific body system(s) affected (heart, brain, kidneys, or eyes) when we consider its effects under the listings. We will also consider any limitations imposed by your hypertension when we consider whether you have an impairment that functionally equals the listings.
3. What is cardiomyopathy and how will we evaluate it? Cardiomyopathy is a disease of the heart muscle. The heart loses its ability to pump blood (heart failure), and in some instances, heart rhythm is disturbed, leading to irregular heartbeats (arrhythmias). Usually, the exact cause of the muscle damage is never found (idiopathic cardiomyopathy). There are various types of cardiomyopathy, which fall into two major categories: Ischemic and nonischemic cardiomyopathy. Ischemic cardiomyopathy typically refers to heart muscle damage that results from coronary artery disease, including heart attacks. Nonischemic cardiomyopathy includes several types: Dilated, hypertrophic, and restrictive. We will evaluate cardiomyopathy under 4.04 in part A, 104.02, 104.05, or 111.06, depending on its effects on you.
4. How will we evaluate valvular heart disease? We will evaluate valvular heart disease under the listing appropriate for its effect on you. Thus, we may use 4.04 in part A, 104.02, 104.05, 104.06, or an appropriate neurological listing in 111.00ff.
5. What do we consider when we evaluate heart transplant recipients?
a. After your heart transplant, we will consider you disabled for 1 year following the surgery because there is a greater likelihood of rejection of the organ and infection during the first year.
b. However, heart transplant patients generally meet our definition of disability before they undergo transplantation. We will determine the onset of your disability based on the facts in your case.
c. We will not assume that you became disabled when your name was placed on a transplant waiting list. This is because you may be placed on a waiting list soon after diagnosis of the cardiac disorder that may eventually require a transplant. Physicians recognize that candidates for transplantation often have to wait months or even years before a suitable donor heart is found, so they place their patients on the list as soon as permitted.
d. When we do a continuing disability review to determine whether you are still disabled, we will evaluate your residual impairment(s), as shown by symptoms, signs, and laboratory findings, including any side effects of medication. We will consider any remaining symptoms, signs, and laboratory findings indicative of cardiac dysfunction in deciding whether medical improvement (as defined in § 416.994a) has occurred.
6. How will we evaluate chronic rheumatic fever or rheumatic heart disease? The diagnosis should be made in accordance with the current revised Jones criteria for guidance in the diagnosis of rheumatic fever. We will evaluate persistence of rheumatic fever activity under 104.13. If you have evidence of chronic heart failure or recurrent arrhythmias associated with rheumatic heart disease, we will use 104.02 or 104.05.
7. What is hyperlipidemia and how will we evaluate it? Hyperlipidemia is the general term for an elevation of any or all of the lipids (fats or cholesterol) in the blood; for example, hypertriglyceridemia, hypercholesterolemia, and hyperlipoproteinemia. These disorders of lipoprotein metabolism and transport can cause defects throughout the body. The effects most likely to interfere with function are those produced by atherosclerosis (narrowing of the arteries) and coronary artery disease. We will evaluate your lipoprotein disorder by considering its effects on you.
8. How will we evaluate Kawasaki disease? We will evaluate Kawasaki disease under the listing appropriate to its effects on you, which may include major coronary artery aneurysm or heart failure. A major coronary artery aneurysm may cause ischemia or arrhythmia, which we will evaluate under 4.04 in part A or 104.05. We will evaluate chronic heart failure under 104.02.
9. What is lymphedema and how will we evaluate it?
a. Lymphedema is edema of the extremities due to a disorder of the lymphatic circulation; at its worst, it is called elephantiasis. Primary lymphedema is caused by abnormal development of lymph vessels and may be present at birth (congenital lymphedema), but more often develops during the teens (lymphedema praecox). Secondary lymphedema is due to obstruction or destruction of normal lymphatic channels due to tumor, surgery, repeated infections, or parasitic infection such as filariasis. Lymphedema most commonly affects one extremity.
b. Lymphedema does not meet the requirements of 4.11 in part A, although it may medically equal the severity of that listing. We will evaluate lymphedema by considering whether the underlying cause meets or medically equals any listing or whether the lymphedema medically equals a cardiovascular listing, such as 4.11, or a musculoskeletal listing, such as 101.02A or 101.03. If no listing is met or medically equaled, we will evaluate any functional limitations imposed by your lymphedema when we consider whether you have an impairment that functionally equals the listings.
10. What is Marfan syndrome and how will we evaluate it?
a. Marfan syndrome is a genetic connective tissue disorder that affects multiple body systems, including the skeleton, eyes, heart, blood vessels, nervous system, skin, and lungs. There is no specific laboratory test to diagnose Marfan syndrome. The diagnosis is generally made by medical history, including family history, physical examination, including an evaluation of the ratio of arm/leg size to trunk size, a slit lamp eye examination, and a heart test(s), such as an echocardiogram. In some cases, a genetic analysis may be useful, but such analyses may not provide any additional helpful information.
b. The effects of Marfan syndrome can range from mild to severe. In most cases, the disorder progresses as you age. Most individuals with Marfan syndrome have abnormalities associated with the heart and blood vessels. Your heart's mitral valve may leak, causing a heart murmur. Small leaks may not cause symptoms, but larger ones may cause shortness of breath, fatigue, and palpitations. Another effect is that the wall of the aorta may be weakened and stretch (aortic dilation). This aortic dilation may tear, dissect, or rupture, causing serious heart problems or sometimes sudden death. We will evaluate the manifestations of your Marfan syndrome under the appropriate body system criteria, such as 4.10 in part A, or if necessary consider the functional limitations imposed by your impairment.
G. Other Evaluation Issues1. What effect does obesity have on the cardiovascular system and how will we evaluate it? Obesity is a medically determinable impairment that is often associated with disorders of the cardiovascular system. Disturbance of this system can be a major cause of disability in children with obesity. Obesity may affect the cardiovascular system because of the increased workload the additional body mass places on the heart. Obesity may make it harder for the chest and lungs to expand. This can mean that the respiratory system must work harder to provide needed oxygen. This in turn would make the heart work harder to pump blood to carry oxygen to the body. Because the body would be working harder at rest, its ability to perform additional work would be less than would otherwise be expected. Thus, the combined effects of obesity with cardiovascular impairments can be greater than the effects of each of the impairments considered separately. We must consider any additional and cumulative effects of obesity when we determine whether you have a severe cardiovascular impairment or a listing-level cardiovascular impairment (or a combination of impairments that medically equals a listing), and when we determine whether your impairment(s) functionally equals the listings.
2. How do we relate treatment to functional status? In general, conclusions about the severity of a cardiovascular impairment cannot be made on the basis of type of treatment rendered or anticipated. The amount of function restored and the time required for improvement after treatment (medical, surgical, or a prescribed program of progressive physical activity) vary with the nature and extent of the disorder, the type of treatment, and other factors. Depending upon the timing of this treatment in relation to the alleged onset date of disability, we may need to defer evaluation of the impairment for a period of up to 3 months from the date treatment began to permit consideration of treatment effects, unless we can make a determination or decision using the evidence we have. See 104.00B4.
3. How do we evaluate impairments that do not meet one of the cardiovascular listings?
a. These listings are only examples of common cardiovascular disorders that we consider severe enough to result in marked and severe functional limitations. If your severe impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
b. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See § 416.926.) If you have a severe impairment(s) that does not meet or medically equal the criteria of a listing, we will consider whether it functionally equals the listings. (See § 416.926a.) When we decide whether you continue to be disabled, we use the rules in § 416.994a.
104.01 Category of Impairments, Cardiovascular System104.02. Chronic heart failure while on a regimen of prescribed treatment, with symptoms and signs described in 104.00C2, and with one of the following:
A. Persistent tachycardia at rest (see Table I);
ORB. Persistent tachypnea at rest (see Table II) or markedly decreased exercise tolerance (see 104.00C2b);
ORC. Growth failure as required in 1 or 2:
1. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate weight-for-length table under 105.08B1; or
2. For children age 2 to attainment of age 18, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate BMI-for-age table under 105.08B2.
104.05 Recurrent arrhythmias, not related to reversible causes such as electrolyte abnormalities or digitalis glycoside or antiarrhythmic drug toxicity, resulting in uncontrolled (see 104.00A3g), recurrent (see 104.00A3c) episodes of cardiac syncope or near syncope (see 104.00E3b), despite prescribed treatment (see 104.00B3 if there is no prescribed treatment), and documented by resting or ambulatory (Holter) electrocardiography, or by other appropriate medically acceptable testing, coincident with the occurrence of syncope or near syncope (see 104.00E3c).
104.06 Congenital heart disease, documented by appropriate medically acceptable imaging (see 104.00A3d) or cardiac catheterization, with one of the following:
A. Cyanotic heart disease, with persistent, chronic hypoxemia as manifested by:
1. Hematocrit of 55 percent or greater on two evaluations 3 months or more apart within a consecutive 12-month period (see 104.00A3e); or
2. Arterial O2 saturation of less than 90 percent in room air, or resting arterial PO2 of 60 Torr or less; or
3. Hypercyanotic spells, syncope, characteristic squatting, or other incapacitating symptoms directly related to documented cyanotic heart disease; or
4. Exercise intolerance with increased hypoxemia on exertion.
ORB. Secondary pulmonary vascular obstructive disease with pulmonary arterial systolic pressure elevated to at least 70 percent of the systemic arterial systolic pressure.
ORC. Symptomatic acyanotic heart disease, with ventricular dysfunction interfering very seriously with the ability to independently initiate, sustain, or complete activities.
ORD. For infants under 12 months of age at the time of filing, with life-threatening congenital heart impairment that will require or already has required surgical treatment in the first year of life, and the impairment is expected to be disabling (because of residual impairment following surgery, or the recovery time required, or both) until the attainment of at least 1 year of age, consider the infant to be under disability until the attainment of at least age 1; thereafter, evaluate impairment severity with reference to the appropriate listing.
104.09 Heart transplant. Consider under a disability for 1 year following surgery; thereafter, evaluate residual impairment under the appropriate listing.
104.13 Rheumatic heart disease, with persistence of rheumatic fever activity manifested by significant murmurs(s), cardiac enlargement or ventricular dysfunction (see 104.00C2a), and other associated abnormal laboratory findings; for example, an elevated sedimentation rate or ECG findings, for 6 months or more in a consecutive 12-month period (see 104.00A3e). Consider under a disability for 18 months from the established onset of impairment, then evaluate any residual impairment(s).
105.00 Digestive SystemA. What kinds of disorders do we consider in the digestive system? Disorders of the digestive system include gastrointestinal hemorrhage, hepatic (liver) dysfunction, inflammatory bowel disease, short bowel syndrome, and malnutrition. They may also lead to complications, such as obstruction, or be accompanied by manifestations in other body systems. Congenital abnormalities involving the organs of the gastrointestinal system may interfere with the ability to maintain adequate nutrition, growth, and development.
B. What documentation do we need? We need a record of your medical evidence, including clinical and laboratory findings. The documentation should include appropriate medically acceptable imaging studies and reports of endoscopy, operations, and pathology, as appropriate to each listing, to document the severity and duration of your digestive disorder. We may also need assessments of your growth and development. Medically acceptable imaging includes, but is not limited to, x-ray imaging, sonography, computerized axial tomography (CAT scan), magnetic resonance imaging (MRI), and radionuclide scans. Appropriate means that the technique used is the proper one to support the evaluation and diagnosis of the disorder. The findings required by these listings must occur within the period we are considering in connection with your application or continuing disability review.
C. How do we consider the effects of treatment?
1. Digestive disorders frequently respond to medical or surgical treatment; therefore, we generally consider the severity and duration of these disorders within the context of the prescribed treatment.
2. We assess the effects of treatment, including medication, therapy, surgery, or any other form of treatment you receive, by determining if there are improvements in the symptoms, signs, and laboratory findings of your digestive disorder. We also assess any side effects of your treatment that may further limit your functioning.
3. To assess the effects of your treatment, we may need information about:
a. The treatment you have been prescribed (for example, the type of medication or therapy, or your use of parenteral (intravenous) nutrition or supplemental enteral nutrition via a gastrostomy);
b. The dosage, method, and frequency of administration;
c. Your response to the treatment;
d. Any adverse effects of such treatment; and
e. The expected duration of the treatment.
4. Because the effects of treatment may be temporary or long-term, in most cases we need information about the impact of your treatment, including its expected duration and side effects, over a sufficient period of time to help us assess its outcome. When adverse effects of treatment contribute to the severity of your impairment(s), we will consider the duration or expected duration of the treatment when we assess the duration of your impairment(s).
5. If you need parenteral (intravenous) nutrition or supplemental enteral nutrition via a gastrostomy to avoid debilitating complications of a digestive disorder, this treatment will not, in itself, indicate that you have marked and severe functional limitations. The exceptions are 105.07, short bowel syndrome, and 105.10, for children who have not attained age 3 and who require supplemental daily enteral feedings via a gastrostomy (see 105.00F and 105.00H).
6. If you have not received ongoing treatment or have not had an ongoing relationship with the medical community despite the existence of a severe impairment(s), we will evaluate the severity and duration of your digestive impairment on the basis of current medical and other evidence in your case record. If you have not received treatment, you may not be able to show an impairment that meets the criteria of one of the digestive system listings, but your digestive impairment may medically equal a listing or functionally equal the listings.
D. How do we evaluate chronic liver disease?
1. General. Chronic liver disease is characterized by liver cell necrosis, inflammation, or scarring (fibrosis or cirrhosis), due to any cause, that persists for more than 6 months. Chronic liver disease may result in portal hypertension, cholestasis (suppression of bile flow), extrahepatic manifestations, or liver cancer. (We evaluate liver cancer under 113.03.) Significant loss of liver function may be manifested by hemorrhage from varices or portal hypertensive gastropathy, ascites (accumulation of fluid in the abdominal cavity), hydrothorax (ascitic fluid in the chest cavity), or encephalopathy. There can also be progressive deterioration of laboratory findings that are indicative of liver dysfunction. Liver transplantation is the only definitive cure for end stage liver disease (ESLD).
2. Examples of chronic liver disease include, but are not limited to, biliary atresia, chronic hepatitis, non-alcoholic steatohepatitis (NASH), primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), autoimmune hepatitis, hemochromatosis, drug-induced liver disease, Wilson's disease, and serum alpha-1 antitrypsin deficiency. Children can also have congenital abnormalities of abdominal organs or inborn metabolic disorders that result in chronic liver disease. Acute hepatic injury is frequently reversible as in viral, drug-induced, toxin-induced, and ischemic hepatitis. In the absence of evidence of a chronic impairment, episodes of acute liver disease do not meet 105.05.
3. Manifestations of chronic liver disease.
a. Symptoms may include, but are not limited to, pruritis (itching), fatigue, nausea, loss of appetite, or sleep disturbances. Children can also have associated developmental delays or poor school performance. Symptoms of chronic liver disease may have a poor correlation with the severity of liver disease and functional ability.
b. Signs may include, but are not limited to, jaundice, enlargement of the liver and spleen, ascites, peripheral edema, and altered mental status.
c. Laboratory findings may include, but are not limited to, increased liver enzymes, increased serum total bilirubin, increased ammonia levels, decreased serum albumin, and abnormal coagulation studies, such as increased International Normalized Ratio (INR) or decreased platelet counts. Abnormally low serum albumin or elevated INR levels indicate loss of synthetic liver function, with increased likelihood of cirrhosis and associated complications. However, other abnormal lab tests, such as liver enzymes, serum total bilirubin, or ammonia levels, may have a poor correlation with the severity of liver disease and functional ability. A liver biopsy may demonstrate the degree of liver cell necrosis, inflammation, fibrosis, and cirrhosis. If you have had a liver biopsy, we will make every reasonable effort to obtain the results; however, we will not purchase a liver biopsy. Imaging studies (CAT scan, ultrasound, MRI) may show the size and consistency (fatty liver, scarring) of the liver and document ascites (see 105.00D6).
4. Chronic viral hepatitis infections.
a. General.
(i) Chronic viral hepatitis infections are commonly caused by hepatitis C virus (HCV), and to a lesser extent, hepatitis B virus (HBV). Usually, these are slowly progressive disorders that persist over many years during which the symptoms and signs are typically nonspecific, intermittent, and mild (for example, fatigue, difficulty with concentration, or right upper quadrant pain). Laboratory findings (liver enzymes, imaging studies, liver biopsy pathology) and complications are generally similar in HCV and HBV. The spectrum of these chronic viral hepatitis infections ranges widely and includes an asymptomatic state; insidious disease with mild to moderate symptoms associated with fluctuating liver tests; extrahepatic manifestations; cirrhosis, both compensated and decompensated; ESLD with the need for liver transplantation; and liver cancer. Treatment for chronic viral hepatitis infections varies considerably based on age, medication tolerance, treatment response, adverse effects of treatment, and duration of the treatment. Comorbid disorders, such as HIV infection, may accelerate the clinical course of viral hepatitis infection(s) or may result in a poorer response to medical treatment.
(ii) We evaluate all types of chronic viral hepatitis infections under 105.05 or any listing in an affected body system(s). If your impairment(s) does not meet or medically equal a listing, we will consider the effects of your hepatitis when we assess whether your impairment(s) functionally equals the listings.
b. Chronic hepatitis B virus (HBV) infection.
(i) Chronic HBV infection can be diagnosed by the detection of hepatitis B surface antigen (HBsAg) or hepatitis B virus DNA (HBV DNA) in the blood for at least 6 months. In addition, detection of the hepatitis B e antigen (HBeAg) suggests an increased likelihood of progression to cirrhosis, ESLD, and hepatocellular carcinoma. (HBeAg may also be referred to as “hepatitis B early antigen” or “hepatitis B envelope antigen.”)
(ii) The therapeutic goal of treatment is to suppress HBV replication and thereby prevent progression to cirrhosis, ESLD, and hepatocellular carcinoma. Treatment usually includes interferon injections, oral antiviral agents, or a combination of both. Common adverse effects of treatment are the same as noted in 105.00D4c(ii) for HCV, and generally end within a few days after treatment is discontinued.
c. Chronic hepatitis C virus (HCV) infection.
(i) Chronic HCV infection is diagnosed by the detection of hepatitis C viral RNA in the blood for at least 6 months. Documentation of the therapeutic response to treatment is also monitored by the quantitative assay of serum HCV RNA (“HCV viral load”). Treatment usually includes a combination of interferon injections and oral ribavirin; whether a therapeutic response has occurred is usually assessed after 12 weeks of treatment by checking the HCV viral load. If there has been a substantial reduction in HCV viral load (also known as early viral response, or EVR), this reduction is predictive of a sustained viral response with completion of treatment. Combined therapy is commonly discontinued after 12 weeks when there is no early viral response, since in that circumstance there is little chance of obtaining a sustained viral response (SVR). Otherwise, treatment is usually continued for a total of 48 weeks.
(ii) Combined interferon and ribavirin treatment may have significant adverse effects that may require dosing reduction, planned interruption of treatment, or discontinuation of treatment. Adverse effects may include: Anemia (ribavirin-induced hemolysis), neutropenia, thrombocytopenia, fever, cough, fatigue, myalgia, arthralgia, nausea, loss of appetite, pruritis, and insomnia. Behavioral side effects may also occur. Influenza-like symptoms are generally worse in the first 4 to 6 hours after each interferon injection and during the first weeks of treatment. Adverse effects generally end within a few days after treatment is discontinued.
d. Extrahepatic manifestations of HBV and HCV. In addition to their hepatic manifestations, both HBV and HCV may have significant extrahepatic manifestations in a variety of body systems. These include, but are not limited to: Keratoconjunctivitis (sicca syndrome), glomerulonephritis, skin disorders (for example, lichen planus, porphyria cutanea tarda), neuropathy, and immune dysfunction (for example, cryoglobulinemia, Sjögren's syndrome, and vasculitis). The extrahepatic manifestations of HBV and HCV may not correlate with the severity of your hepatic impairment. If your impairment(s) does not meet or medically equal a listing in an affected body system(s), we will consider the effects of your extrahepatic manifestations when we determine whether your impairment(s) functionally equals the listings.
5. Gastrointestinal hemorrhage (105.02 and 105.05A). Gastrointestinal hemorrhaging can result in hematemesis (vomiting of blood), melena (tarry stools), or hematochezia (bloody stools). Under 105.02, the required transfusions of at least 10 cc of blood/kg of body weight must be at least 30 days apart and occur at least three times during a consecutive 6-month period. Under 105.05A, hemodynamic instability is diagnosed with signs such as pallor (pale skin), diaphoresis (profuse perspiration), rapid pulse, low blood pressure, postural hypotension (pronounced fall in blood pressure when arising to an upright position from lying down) or syncope (fainting). Hemorrhaging that results in hemodynamic instability is potentially life-threatening and therefore requires hospitalization for transfusion and supportive care. Under 105.05A, we require only one hospitalization for transfusion of at least 10 cc of blood/kg of body weight.
6. Ascites or hydrothorax (105.05B) indicates significant loss of liver function due to chronic liver disease. We evaluate ascites or hydrothorax that is not attributable to other causes under 105.05B. The required findings must be present on at least two evaluations at least 60 days apart within a consecutive 6-month period and despite continuing treatment as prescribed.
7. Spontaneous bacterial peritonitis (105.05C) is an infectious complication of chronic liver disease. It is diagnosed by ascitic peritoneal fluid that is documented to contain an absolute neutrophil count of at least 250 cells/mm 3. The required finding in 105.05C is satisfied with one evaluation documenting peritoneal fluid infection. We do not evaluate other causes of peritonitis that are unrelated to chronic liver disease, such as tuberculosis, malignancy, and perforated bowel, under this listing. We evaluate these other causes of peritonitis under the appropriate body system listings.
8. Hepatorenal syndrome (105.05D) is defined as functional renal failure associated with chronic liver disease in the absence of underlying kidney pathology. Hepatorenal syndrome is documented by elevation of serum creatinine, marked sodium retention, and oliguria (reduced urine output). The requirements of 105.05D are satisfied with documentation of any one of the three laboratory findings on one evaluation. We do not evaluate known causes of renal dysfunction, such as glomerulonephritis, tubular necrosis, drug-induced renal disease, and renal infections, under this listing. We evaluate these other renal impairments under 106.00ff.
9. Hepatopulmonary syndrome (105.05E) is defined as arterial deoxygenation (hypoxemia) that is associated with chronic liver disease due to intrapulmonary arteriovenous shunting and vasodilatation, in the absence of other causes of arterial deoxygenation. Clinical manifestations usually include dyspnea, orthodeoxia (increasing hypoxemia with erect position), platypnea (improvement of dyspnea with flat position), cyanosis, and clubbing. The requirements of 105.05E are satisfied with documentation of any one of the findings on one evaluation. In 105.05E1, we require documentation of the altitude of the testing facility because altitude affects the measurement of arterial oxygenation. We will not purchase the specialized studies described in 105.05E2; however, if you have had these studies at a time relevant to your claim, we will make every reasonable effort to obtain the reports for the purpose of establishing whether your impairment meets 105.05E2.
10. Hepatic encephalopathy (105.05F).
a. General. Hepatic encephalopathy usually indicates severe loss of hepatocellular function. We define hepatic encephalopathy under 105.05F as a recurrent or chronic neuropsychiatric disorder, characterized by abnormal behavior, cognitive dysfunction, altered state of consciousness, and ultimately coma and death. The diagnosis is established by changes in mental status associated with fleeting neurological signs, including “flapping tremor” (asterixis), characteristic electroencephalographic (EEG) abnormalities, or abnormal laboratory values that indicate loss of synthetic liver function. We will not purchase the EEG testing described in 105.05F3b. However, if you have had this test at a time relevant to your claim, we will make every reasonable effort to obtain the report for the purpose of establishing whether your impairment meets 105.05F.
b. Acute encephalopathy. We will not evaluate your acute encephalopathy under 105.05F if it results from conditions other than chronic liver disease, such as vascular events and neoplastic diseases. We will evaluate these other causes of acute encephalopathy under the appropriate body system listings.
11. End stage liver disease (ESLD) documented by scores from the SSA Chronic Liver Disease (SSA CLD) calculation (105.05G1) and SSA Chronic Liver Disease-Pediatric (SSA CLD-P) calculation (105.05G2).
a. SSA CLD score.
(i) If you are age 12 or older, we will use the SSA CLD score to evaluate your ESLD under 105.05G1. We explain how we calculate the SSA CLD score in a(ii) through a(vii) of this section.
(ii) To calculate the SSA CLD score, we use a formula that includes three laboratory values: Serum total bilirubin (mg/dL), serum creatinine (mg/dL), and International Normalized Ratio (INR). The formula for the SSA CLD score calculation is:
9.57 × [Loge (serum creatinine mg/dL)] + 3.78 × [Loge (serum total bilirubin mg/dL)] + 11.2 × [Loge (INR)] + 6.43(iii) When we indicate “Loge” in the formula for the SSA CLD score calculation, we mean the “base e logarithm” or “natural logarithm” (ln) of a numerical laboratory value, not the “base 10 logarithm” or “common logarithm” (log) of the laboratory value, and not the actual laboratory value. For an example of SSA CLD calculation, see 5.00D11c.
(iv) For any SSA CLD score calculation, all of the required laboratory values must have been obtained within 30 days of each other. If there are multiple laboratory values within the 30-day interval for any given laboratory test (serum total bilirubin, serum creatinine, or INR), we will use the highest value for the SSA CLD score calculation. We will round all laboratory values less than 1.0 up to 1.0.
(v) Listing 105.05G requires two SSA CLD scores. The laboratory values for the second SSA CLD score calculation must have been obtained at least 60 days after the latest laboratory value for the first SSA CLD score and within the required 6-month period. We will consider the date of each SSA CLD score to be the date of the first laboratory value used for its calculation.
(vi) If you are in renal failure or on dialysis within a week of any serum creatinine test in the period used for the SSA CLD calculation, we will use a serum creatinine of 4, which is the maximum serum creatinine level allowed in the calculation, to calculate your SSA CLD score.
(vii) If you have the two SSA CLD scores required by 105.05G1, we will find that your impairment meets the criteria of the listing from at least the date of the first SSA CLD score.
b. SSA CLD-P score.
(i) If you have not attained age 12, we will use the SSA CLD-P score to evaluate your ESLD under 105.05G2. We explain how we calculate the SSA CLD-P score in b(ii) through b(vii) of this section.
(ii) To calculate the SSA CLD-P score, we use a formula that includes four parameters: Serum total bilirubin (mg/dL), International Normalized Ratio (INR), serum albumin (g/dL), and whether growth failure is occurring. The formula for the SSA CLD-P score calculation is:
4.80 × [Loge (serum total bilirubin mg/dL)] + 18.57 × [Loge (INR)] −6.87 × [Loge (serum albumin g/dL)] + 6.67 if the child has growth failure (<−2 standard deviations for weight or height)(iii) When we indicate “Loge” in the formula for the SSA CLD-P score calculation, we mean the “base e logarithm” or “natural logarithm” (ln) of a numerical laboratory value, not the “base 10 logarithm” or “common logarithm” (log) of the laboratory value, and not the actual laboratory value. For example, if a female child is 4.0 years old, has a current weight of 13.5 kg (10th percentile for age) and height of 92 cm (less than the third percentile for age), and has laboratory values of serum total bilirubin 2.2 mg/dL, INR 1.0, and serum albumin 3.5 g/dL, we will compute the SSA CLD-P score as follows:
4.80 × [Loge + (serum total bilirubin 2.2 mg/dL) = 0.788] + 18.57 × [Loge (INR 1.0) = 0] −6.87 × [Loge + (serum albumin 3.5 g/dL) = 1.253] + 6.67 ___ = 3.78 + 0 − 8.61 + 6.67 = 1.84, which is then rounded to an SSA CLD-P score of 2(iv) For any SSA CLD-P score calculation, all of the required laboratory values (serum total bilirubin, INR, or serum albumin) must have been obtained within 30 days of each other. We will not purchase INR values for children who have not attained age 12. If there is no INR value for a child under 12 within the applicable time period, we will use an INR value of 1.1 to calculate the SSA CLD-P score. If there are multiple laboratory values within the 30-day interval for any given laboratory test, we will use the highest serum total bilirubin and INR values and the lowest serum albumin value for the SSA CLD-P score calculation. We will round all laboratory values less than 1.0 up to 1.0.
(v) The weight and length/height measurements used for the calculation must be obtained from one evaluation within the same 30-day period as in D11b(iv).
(vi) Listing 105.05G2 requires two SSA CLD-P scores. The laboratory values for the second SSA CLD-P score calculation must have been obtained at least 60 days after the latest laboratory value for the first SSA CLD-P score and within the required 6-month period. We will consider the date of each SSA CLD-P score to be the date of the first laboratory value used for its calculation.
(vii) If you have the two SSA CLD-P scores required by listing 105.05G2, we will find that your impairment meets the criteria of the listing from at least the date of the first SSA CLD-P score.
12. Extrahepatic biliary atresia (EBA) (105.05H) usually presents in the first 2 months of life with persistent jaundice. The impairment meets 105.05H if the diagnosis of EBA is confirmed by liver biopsy or intraoperative cholangiogram that shows obliteration of the extrahepatic biliary tree. EBA is usually surgically treated by portoenterostomy (for example, Kasai procedure). If this surgery is not performed in the first months of life or is not completely successful, liver transplantation is indicated. If you have had a liver transplant, we will evaluate your impairment under 105.09.
13. Liver transplantation (105.09) may be performed for metabolic liver disease, progressive liver failure, life-threatening complications of liver disease, hepatic malignancy, and acute fulminant hepatitis (viral, drug-induced, or toxin-induced). We will consider you to be disabled for 1 year from the date of the transplantation. Thereafter, we will evaluate your residual impairment(s) by considering the adequacy of post-transplant liver function, the requirement for post-transplant antiviral therapy, the frequency and severity of rejection episodes, comorbid complications, and all adverse treatment effects.
E. How do we evaluate inflammatory bowel disease (IBD)?
1. Inflammatory bowel disease (105.06) includes, but is not limited to, Crohn's disease and ulcerative colitis. These disorders, while distinct entities, share many clinical, laboratory, and imaging findings, as well as similar treatment regimens. Remissions and exacerbations of variable duration are the hallmark of IBD. Crohn's disease may involve the entire alimentary tract from the mouth to the anus in a segmental, asymmetric fashion. Obstruction, stenosis, fistulization, perineal involvement, and extraintestinal manifestations are common. Crohn's disease is rarely curable and recurrence may be a lifelong problem, even after surgical resection. In contrast, ulcerative colitis only affects the colon. The inflammatory process may be limited to the rectum, extend proximally to include any contiguous segment, or involve the entire colon. Ulcerative colitis may be cured by total colectomy.
2. Symptoms and signs of IBD include diarrhea, fecal incontinence, rectal bleeding, abdominal pain, fatigue, fever, nausea, vomiting, arthralgia, abdominal tenderness, palpable abdominal mass (usually inflamed loops of bowel) and perineal disease. You may also have signs or laboratory findings indicating malnutrition, such as weight loss, edema, anemia, hypoalbuminemia, hypokalemia, hypocalcemia, or hypomagnesemia.
3. IBD may be associated with significant extraintestinal manifestations in a variety of body systems. These include, but are not limited to, involvement of the eye (for example, uveitis, episcleritis, iritis); hepatobiliary disease (for example, gallstones, primary sclerosing cholangitis); urologic disease (for example, kidney stones, obstructive hydronephrosis); skin involvement (for example, erythema nodosum, pyoderma gangrenosum); or non-destructive inflammatory arthritis. You may also have associated thromboembolic disorders or vascular disease. These manifestations may not correlate with the severity of your IBD. If your impairment does not meet any of the criteria of 105.06, we will consider the effects of your extraintestinal manifestations in determining whether you have an impairment(s) that meets or medically equals another listing, and we will also consider the effects of your extraintestinal manifestations when we determine whether your impairment(s) functionally equals the listings.
4. Surgical diversion of the intestinal tract, including ileostomy and colostomy, does not very seriously interfere with age-appropriate functioning if you are able to maintain adequate nutrition and function of the stoma. However, if you are not able to maintain adequate nutrition, we will evaluate your impairment under 105.08.
F. How do we evaluate short bowel syndrome (SBS)?
1. Short bowel syndrome (105.07) is a disorder that occurs when congenital intestinal abnormalities, ischemic vascular insults (for example, necrotizing enterocolitis, volvulus), trauma, or IBD complications require surgical resection of more than one-half of the small intestine, resulting in the loss of intestinal absorptive surface and a state of chronic malnutrition. The management of SBS requires long-term parenteral nutrition via an indwelling central venous catheter (central line); the process is often referred to as hyperalimentation or total parenteral nutrition (TPN). Children with SBS can also feed orally, with variable amounts of nutrients being absorbed through their remaining intestine. Over time, some of these children can develop additional intestinal absorptive surface, and may ultimately be able to be weaned off their parenteral nutrition.
2. Your impairment will continue to meet 105.07 as long as you remain dependent on daily parenteral nutrition via a central venous catheter for most of your nutritional requirements. Long-term complications of SBS and parenteral nutrition include abnormal growth rates, central line infections (with or without septicemia), thrombosis, hepatotoxicity, gallstones, and loss of venous access sites. Intestinal transplantation is the only definitive treatment for children with SBS who remain chronically dependent on parenteral nutrition.
3. To document SBS, we need a copy of the operative report of intestinal resection, the summary of the hospitalization(s) including: Details of the surgical findings, medically appropriate postoperative imaging studies that reflect the amount of your residual small intestine, or if we cannot get one of these reports, other medical reports that include details of the surgical findings. We also need medical documentation that you are dependent on daily parenteral nutrition to provide most of your nutritional requirements.
G. How do we evaluate growth failure due to any digestive disorder?
1. To evaluate growth failure due to any digestive disorder, we require documentation of the laboratory findings of chronic nutritional deficiency described in 105.08A and the growth measurements in 105.08B within the same consecutive 12-month period. The dates of laboratory findings may be different from the dates of growth measurements.
2. Under 105.08B, we evaluate a child's growth failure by using the appropriate table for age and gender.
a. For children from birth to attainment of age 2, we use the weight-for-length table (see Table I or Table II).
b. For children age 2 to attainment of age 18, we use the body mass index (BMI)-for-age table (see Tables III or IV).
c. BMI is the ratio of a child's weight to the square of the child's height. We calculate BMI using one of the following formulas:
English Formula BMI = [Weight in Pounds/(Height in Inches × Height in Inches)] × 703 Metric Formulas BMI = Weight in Kilograms/(Height in Meters × Height in Meters) BMI = [Weight in Kilograms/(Height in Centimeters × Height in Centimeters)] × 10,000H. How do we evaluate the need for supplemental daily enteral feeding via a gastrostomy?
1. General. Infants and young children may have anatomical, neurological, or developmental disorders that interfere with their ability to feed by mouth, resulting in inadequate caloric intake to meet their growth needs. These disorders frequently result in the medical necessity to supplement caloric intake and to bypass the anatomical feeding route of mouth-throat-esophagus into the stomach.
2. Children who have not attained age 3 and who require supplemental daily enteral nutrition via a feeding gastrostomy meet 105.10 regardless of the medical reason for the gastrostomy. Thereafter, we evaluate growth impairment under 100.02, malnutrition under 105.08, or other medical or developmental disorder(s) (including the disorder(s) that necessitated gastrostomy placement) under the appropriate listing(s).
I. How do we evaluate esophageal stricture or stenosis? Esophageal stricture or stenosis (narrowing) from congenital atresia (absence or abnormal closure of a tubular body organ) or destructive esophagitis may result in malnutrition or the need for gastrostomy placement, which we evaluate under 105.08 or 105.10. Esophageal stricture or stenosis may also result in complications such as pneumonias due to frequent aspiration, or difficulty in maintaining nutritional status short of listing-level severity. While none of these complications may be of such severity that they would meet the criteria of another listing, the combination of impairments may medically equal the severity of a listing or functionally equal the listings.
J. What do we mean by the phrase “consider under a disability for 1 year”? We use the phrase “consider under a disability for 1 year” following a specific event in 105.02, 105.05A, and 105.09 to explain how long your impairment can meet the requirements of those particular listings. This phrase does not refer to the date on which your disability began, only to the date on which we must reevaluate whether your impairment continues to meet a listing or is otherwise disabling. For example, if you have received a liver transplant, you may have become disabled before the transplant because of chronic liver disease. Therefore, we do not restrict our determination of the onset of disability to the date of the specified event. We will establish an onset date earlier than the date of the specified event if the evidence in your case record supports such a finding.
K. How do we evaluate impairments that do not meet one of the digestive disorder listings?
1. These listings are only examples of common digestive disorders that we consider severe enough to result in marked and severe functional limitations. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system. For example:
a. If you have hepatitis B or C and you are depressed, we will evaluate your impairment under 112.04.
b. If you have multiple congenital abnormalities, we will evaluate your impairment(s) under the criteria in the listings for impairments that affect multiple body systems (110.00) or the criteria of listings in other affected body systems.
c. If you have digestive disorders that interfere with intake, digestion, or absorption of nutrition, and result in a reduction in your rate of growth, and your impairment does not satisfy the criteria in the malnutrition listing (105.08), we will evaluate your impairment under the growth impairment listings (100.00).
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See § 416.926.) If your impairment(s) does not meet or medically equal a listing, you may or may not have an impairment(s) that functionally equals the listings. (See § 416.926a.) When we decide whether you continue to be disabled, we use the rules in § 416.994a.
105.01 Category of Impairments, Digestive System
105.02 Gastrointestinal hemorrhaging from any cause, requiring blood transfusion (with or without hospitalization) of at least 10 cc of blood/kg of body weight, and occurring at least three times during a consecutive 6-month period. The transfusions must be at least 30 days apart within the 6-month period. Consider under a disability for 1 year following the last documented transfusion; thereafter, evaluate the residual impairment(s).
105.03-105.04 [Reserved]
105.05 Chronic liver disease, with:
A. Hemorrhaging from esophageal, gastric, or ectopic varices or from portal hypertensive gastropathy, demonstrated by endoscopy, x-ray, or other appropriate medically acceptable imaging, resulting in hemodynamic instability as defined in 105.00D5, and requiring hospitalization for transfusion of at least 10 cc of blood/kg of body weight. Consider under a disability for 1 year following the last documented transfusion; thereafter, evaluate the residual impairment(s).
ORB. Ascites or hydrothorax not attributable to other causes, despite continuing treatment as prescribed, present on at least two evaluations at least 60 days apart within a consecutive 6-month period. Each evaluation must be documented by:
1. Paracentesis or thoracentesis; or
2. Appropriate medically acceptable imaging or physical examination and one of the following:
a. Serum albumin of 3.0 g/dL or less; or
b. International Normalized Ratio (INR) of at least 1.5.
ORC. Spontaneous bacterial peritonitis with peritoneal fluid containing an absolute neutrophil count of at least 250 cells/mm 3.
ORD. Hepatorenal syndrome as described in 105.00D8, with one of the following:
1. Serum creatinine elevation of at least 2 mg/dL; or
2. Oliguria with 24-hour urine output less than 1 mL/kg/hr; or
3. Sodium retention with urine sodium less than 10 mEq per liter.
ORE. Hepatopulmonary syndrome as described in 105.00D9, with:
1. Arterial oxygenation (PaO2,) on room air of:
a. 60 mm Hg or less, at test sites less than 3000 feet above sea level, or
b. 55 mm Hg or less, at test sites from 3000 to 6000 feet, or
c. 50 mm Hg or less, at test sites above 6000 feet; or
2. Documentation of intrapulmonary arteriovenous shunting by contrast-enhanced echocardiography or macroaggregated albumin lung perfusion scan.
ORF. Hepatic encephalopathy as described in 105.00D10, with 1 and either 2 or 3:
1. Documentation of abnormal behavior, cognitive dysfunction, changes in mental status, or altered state of consciousness (for example, confusion, delirium, stupor, or coma), present on at least two evaluations at least 60 days apart within a consecutive 6-month period; and
2. History of transjugular intrahepatic portosystemic shunt (TIPS) or any surgical portosystemic shunt; or
3. One of the following occurring on at least two evaluations at least 60 days apart within the same consecutive 6-month period as in F1:
a. Asterixis or other fluctuating physical neurological abnormalities; or
b. Electroencephalogram (EEG) demonstrating triphasic slow wave activity; or
c. Serum albumin of 3.0 g/dL or less; or
d. International Normalized Ratio (INR) of 1.5 or greater.
ORG. End Stage Liver Disease, with:
1. For children 12 years of age or older, SSA CLD scores of 22 or greater calculated as described in 105.00D11a. Consider under a disability from at least the date of the first score.
2. For children who have not attained age 12, SSA CLD-P scores of 11 or greater calculated as described in 105.00D11b. Consider under a disability from at least the date of the first score.
ORH. Extrahepatic biliary atresia as diagnosed on liver biopsy or intraoperative cholangiogram. Consider under a disability for 1 year following the diagnosis; thereafter, evaluate the residual liver function.
105.06 Inflammatory bowel disease (IBD) documented by endoscopy, biopsy, appropriate medically acceptable imaging, or operative findings with:
A. Obstruction of stenotic areas (not adhesions) in the small intestine or colon with proximal dilatation, confirmed by appropriate medically acceptable imaging or in surgery, requiring hospitalization for intestinal decompression or for surgery, and occurring on at least two occasions at least 60 days apart within a consecutive 6-month period;
ORB. Two of the following despite continuing treatment as prescribed and occurring within the same consecutive 6-month period:
1. Anemia with hemoglobin less than 10.0 g/dL, present on at least two evaluations at least 60 days apart; or
2. Serum albumin of 3.0 g/dL or less, present on at least two evaluations at least 60 days apart; or
3. Clinically documented tender abdominal mass palpable on physical examination with abdominal pain or cramping that is not completely controlled by prescribed narcotic medication, present on at least two evaluations at least 60 days apart; or
4. Perineal disease with a draining abscess or fistula, with pain that is not completely controlled by prescribed narcotic medication, present on at least two evaluations at least 60 days apart; or
5. Need for supplemental daily enteral nutrition via a gastrostomy or daily parenteral nutrition via a central venous catheter. (See 105.10 for children who have not attained age 3.)
105.07 Short bowel syndrome (SBS), due to surgical resection of more than one-half of the small intestine, with dependence on daily parenteral nutrition via a central venous catheter (see 105.00F).
105.08 Growth failure due to any digestive disorder (see 105.00G), documented by A and B:
A. Chronic nutritional deficiency present on at least two evaluations at least 60 days apart within a consecutive 12-month period documented by one of the following:
1. Anemia with hemoglobin less than 10.0 g/dL; or
2. Serum albumin of 3.0 g/dL or less;
ANDB. Growth failure as required in 1 or 2:
1. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on Table I or Table II; or
Table I - Males Birth to Attainment of Age 2
[Third Percentile Values for Weight-for-Length]
| Length (centimeters) |
Weight (kilograms) |
Length (centimeters) |
Weight (kilograms) |
Length (centimeters) |
Weight (kilograms) |
|---|---|---|---|---|---|
| 45.0 | 1.597 | 64.5 | 6.132 | 84.5 | 10.301 |
| 45.5 | 1.703 | 65.5 | 6.359 | 85.5 | 10.499 |
| 46.5 | 1.919 | 66.5 | 6.584 | 86.5 | 10.696 |
| 47.5 | 2.139 | 67.5 | 6.807 | 87.5 | 10.895 |
| 48.5 | 2.364 | 68.5 | 7.027 | 88.5 | 11.095 |
| 49.5 | 2.592 | 69.5 | 7.245 | 89.5 | 11.296 |
| 50.5 | 2.824 | 70.5 | 7.461 | 90.5 | 11.498 |
| 51.5 | 3.058 | 71.5 | 7.674 | 91.5 | 11.703 |
| 52.5 | 3.294 | 72.5 | 7.885 | 92.5 | 11.910 |
| 53.5 | 3.532 | 73.5 | 8.094 | 93.5 | 12.119 |
| 54.5 | 3.771 | 74.5 | 8.301 | 94.5 | 12.331 |
| 55.5 | 4.010 | 75.5 | 8.507 | 95.5 | 12.546 |
| 56.5 | 4.250 | 76.5 | 8.710 | 96.5 | 12.764 |
| 57.5 | 4.489 | 77.5 | 8.913 | 97.5 | 12.987 |
| 58.5 | 4.728 | 78.5 | 9.113 | 98.5 | 13.213 |
| 59.5 | 4.966 | 79.5 | 9.313 | 99.5 | 13.443 |
| 60.5 | 5.203 | 80.5 | 9.512 | 100.5 | 13.678 |
| 61.5 | 5.438 | 81.5 | 9.710 | 101.5 | 13.918 |
| 62.5 | 5.671 | 82.5 | 9.907 | 102.5 | 14.163 |
| 63.5 | 5.903 | 83.5 | 10.104 | 103.5 | 14.413 |
Table II - Females Birth to Attainment of Age 2
[Third percentile values for weight-for-length]
| Length (centimeters) |
Weight (kilograms) |
Length (centimeters) |
Weight (kilograms) |
Length (centimeters) |
Weight (kilograms) |
|---|---|---|---|---|---|
| 45.0 | 1.613 | 64.5 | 5.985 | 84.5 | 10.071 |
| 45.5 | 1.724 | 65.5 | 6.200 | 85.5 | 10.270 |
| 46.5 | 1.946 | 66.5 | 6.413 | 86.5 | 10.469 |
| 47.5 | 2.171 | 67.5 | 6.625 | 87.5 | 10.670 |
| 48.5 | 2.397 | 68.5 | 6.836 | 88.5 | 10.871 |
| 49.5 | 2.624 | 69.5 | 7.046 | 89.5 | 11.074 |
| 50.5 | 2.852 | 70.5 | 7.254 | 90.5 | 11.278 |
| 51.5 | 3.081 | 71.5 | 7.461 | 91.5 | 11.484 |
| 52.5 | 3.310 | 72.5 | 7.667 | 92.5 | 11.691 |
| 53.5 | 3.538 | 73.5 | 7.871 | 93.5 | 11.901 |
| 54.5 | 3.767 | 74.5 | 8.075 | 94.5 | 12.112 |
| 55.5 | 3.994 | 75.5 | 8.277 | 95.5 | 12.326 |
| 56.5 | 4.220 | 76.5 | 8.479 | 96.5 | 12.541 |
| 57.5 | 4.445 | 77.5 | 8.679 | 97.5 | 12.760 |
| 58.5 | 4.669 | 78.5 | 8.879 | 98.5 | 12.981 |
| 59.5 | 4.892 | 79.5 | 9.078 | 99.5 | 13.205 |
| 60.5 | 5.113 | 80.5 | 9.277 | 100.5 | 13.431 |
| 61.5 | 5.333 | 81.5 | 9.476 | 101.5 | 13.661 |
| 62.5 | 5.552 | 82.5 | 9.674 | 102.5 | 13.895 |
| 63.5 | 5.769 | 83.5 | 9.872 | 103.5 | 14.132 |
2. For children age 2 to attainment of age 18, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on Table III or Table IV.
Table III - Males Age 2 to Attainment of Age 18
[Third Percentile Values for BMI-for-Age]
| Age (yrs. and mos.) |
BMI | Age (yrs. and mos.) |
BMI | Age (yrs. and mos.) |
BMI |
|---|---|---|---|---|---|
| 2.0 to 2.1 | 14.5 | 10.11 to 11.2 | 14.3 | 14.9 to 14.10 | 16.1 |
| 2.2 to 2.4 | 14.4 | 11.3 to 11.5 | 14.4 | 14.11 to 15.0 | 16.2 |
| 2.5 to 2.7 | 14.3 | 11.6 to 11.8 | 14.5 | 15.1 to 15.3 | 16.3 |
| 2.8 to 2.11 | 14.2 | 11.9 to 11.11 | 14.6 | 15.4 to 15.5 | 16.4 |
| 3.0 to 3.2 | 14.1 | 12.0 to 12.1 | 14.7 | 15.6 to 15.7 | 16.5 |
| 3.3 to 3.6 | 14.0 | 12.2 to 12.4 | 14.8 | 15.8 to 15.9 | 16.6 |
| 3.7 to 3.11 | 13.9 | 12.5 to 12.7 | 14.9 | 15.10 to 15.11 | 16.7 |
| 4.0 to 4.5 | 13.8 | 12.8 to 12.9 | 15.0 | 16.0 to 16.1 | 16.8 |
| 4.6 to 5.0 | 13.7 | 12.10 to 13.0 | 15.1 | 16.2 to 16.3 | 16.9 |
| 5.1 to 6.0 | 13.6 | 13.1 to 13.2 | 15.2 | 16.4 to 16.5 | 17.0 |
| 6.1 to 7.6 | 13.5 | 13.3 to 13.4 | 15.3 | 16.6 to 16.8 | 17.1 |
| 7.7 to 8.6 | 13.6 | 13.5 to 13.7 | 15.4 | 16.9 to 16.10 | 17.2 |
| 8.7 to 9.1 | 13.7 | 13.8 to 13.9 | 15.5 | 16.11 to 17.0 | 17.3 |
| 9.2 to 9.6 | 13.8 | 13.10 to 13.11 | 15.6 | 17.1 to 17.2 | 17.4 |
| 9.7 to 9.11 | 13.9 | 14.0 to 14.1 | 15.7 | 17.3 to 17.5 | 17.5 |
| 10.0 to 10.3 | 14.0 | 14.2 to 14.4 | 15.8 | 17.6 to 17.7 | 17.6 |
| 10.4 to 10.7 | 14.1 | 14.5 to 14.6 | 15.9 | 17.8 to 17.9 | 17.7 |
| 10.8 to 10.10 | 14.2 | 14.7 to 14.8 | 16.0 | 17.10 to 17.11 | 17.8 |
Table IV - Females Age 2 to Attainment of Age 18
[Third Percentile Values for BMI-for-Age]
| Age (yrs. and mos.) |
BMI | Age (yrs. and mos.) |
BMI | Age (yrs. and mos.) |
BMI |
|---|---|---|---|---|---|
| 2.0 to 2.2 | 14.1 | 10.8 to 10.10 | 14.0 | 14.3 to 14.5 | 15.6 |
| 2.3 to 2.6 | 14.0 | 10.11 to 11.2 | 14.1 | 14.6 to 14.7 | 15.7 |
| 2.7 to 2.10 | 13.9 | 11.3 to 11.5 | 14.2 | 14.8 to 14.9 | 15.8 |
| 2.11 to 3.2 | 13.8 | 11.6 to 11.7 | 14.3 | 14.10 to 15.0 | 15.9 |
| 3.3 to 3.6 | 13.7 | 11.8 to 11.10 | 14.4 | 15.1 to 15.2 | 16.0 |
| 3.7 to 3.11 | 13.6 | 11.11 to 12.1 | 14.5 | 15.3 to 15.5 | 16.1 |
| 4.0 to 4.4 | 13.5 | 12.2 to 12.4 | 14.6 | 15.6 to 15.7 | 16.2 |
| 4.5 to 4.11 | 13.4 | 12.5 to 12.6 | 14.7 | 15.8 to 15.10 | 16.3 |
| 5.0 to 5.9 | 13.3 | 12.7 to 12.9 | 14.8 | 15.11 to 16.0 | 16.4 |
| 5.10 to 7.6 | 13.2 | 12.10 to 12.11 | 14.9 | 16.1 to 16.3 | 16.5 |
| 7.7 to 8.4 | 13.3 | 13.0 to 13.2 | 15.0 | 16.4 to 16.6 | 16.6 |
| 8.5 to 8.10 | 13.4 | 13.3 to 13.4 | 15.1 | 16.7 to 16.9 | 16.7 |
| 8.11 to 9.3 | 13.5 | 13.5 to 13.7 | 15.2 | 16.10 to 17.0 | 16.8 |
| 9.4 to 9.8 | 13.6 | 13.8 to 13.9 | 15.3 | 17.1 to 17.3 | 16.9 |
| 9.9 to 10.0 | 13.7 | 13.10 to 14.0 | 15.4 | 17.4 to 17.7 | 17.0 |
| 10.1 to 10.4 | 13.8 | 14.1 to 14.2 | 15.5 | 17.8 to 17.11 | 17.1 |
| 10.5 to 10.7 | 13.9 |
105.09 Liver transplantation. Consider under a disability for 1 year following the date of transplantation; thereafter, evaluate the residual impairment(s) (see 105.00D13 and 105.00J).
105.10 Need for supplemental daily enteral feeding via a gastrostomy due to any cause, for children who have not attained age 3; thereafter, evaluate the residual impairment(s) (see 105.00H).
106.00 Genitourinary Disorders A. Which disorders do we evaluate under these listings?We evaluate genitourinary disorders resulting in chronic kidney disease (CKD). Examples of such disorders include chronic glomerulonephritis, hypertensive nephropathy, diabetic nephropathy, chronic obstructive uropathy, and hereditary nephropathies. We also evaluate nephrotic syndrome due to glomerular dysfunction, and congenital genitourinary disorders, such as ectopic ureter, exstrophic urinary bladder, urethral valves, and Eagle-Barrett syndrome (prune belly syndrome), under these listings.
B. What evidence do we need?1. We need evidence that documents the signs, symptoms, and laboratory findings of your CKD. This evidence should include reports of clinical examinations, treatment records, and documentation of your response to treatment. Laboratory findings, such as serum creatinine or serum albumin levels, may document your kidney function. We generally need evidence covering a period of at least 90 days unless we can make a fully favorable determination or decision without it.
2. Estimated glomerular filtration rate (eGFR). The eGFR is an estimate of the filtering capacity of the kidneys that takes into account serum creatinine concentration and other variables, such as your age, gender, and body size. If your medical evidence includes eGFR findings, we will consider them when we evaluate your CKD under 106.05.
3. Kidney or bone biopsy. If you have had a kidney or bone biopsy, we need a copy of the pathology report. When we cannot get a copy of the pathology report, we will accept a statement from an acceptable medical source verifying that a biopsy was performed and describing the results.
C. What other factors do we consider when we evaluate your genitourinary disorder?1. Chronic hemodialysis or peritoneal dialysis.
a. Dialysis is a treatment for CKD that uses artificial means to remove toxic metabolic byproducts from the blood. Hemodialysis uses an artificial kidney machine to clean waste products from the blood; peritoneal dialysis uses a dialyzing solution that is introduced into and removed from the abdomen (peritoneal cavity) either continuously or intermittently. Under 106.03, your ongoing dialysis must have lasted or be expected to last for a continuous period of at least 12 months. To satisfy the requirement in 106.03, we will accept a report from an acceptable medical source that describes your CKD and your current dialysis, and indicates that your dialysis will be ongoing.
b. If you are undergoing chronic hemodialysis or peritoneal dialysis, your CKD may meet our definition of disability before you started dialysis. We will determine the onset of your disability based on the facts in your case record.
2. Kidney transplant.
a. If you receive a kidney transplant, we will consider you to be disabled under 106.04 for 1 year from the date of transplant. After that, we will evaluate your residual impairment(s) by considering your post-transplant function, any rejection episodes you have had, complications in other body systems, and any adverse effects related to ongoing treatment.
b. If you received a kidney transplant, your CKD may meet our definition of disability before you received the transplant. We will determine the onset of your disability based on the facts in your case record.
3. Anasarca (generalized massive edema or swelling). Under 106.06B, we need a description of the extent of edema, including pretibial (in front of the tibia), periorbital (around the eyes), or presacral (in front of the sacrum) edema. We also need a description of any ascites, pleural effusion, or pericardial effusion.
4. Congenital genitourinary disorder. Procedures such as diagnostic cystoscopy or circumcision do not satisfy the requirement for urologic surgical procedures in 106.07.
5. Growth failure due to any chronic renal disease.
a. To evaluate growth failure due to any chronic renal disease, we require documentation of the laboratory findings described in 106.08A and the growth measurements in 106.08B within the same consecutive 12-month period. The dates of laboratory findings may be different from the dates of growth measurements.
b. Under 106.08B, we use the appropriate table(s) under 105.08B in the digestive system to determine whether a child's growth is less than the third percentile.
(i) For children from birth to attainment of age 2, we use the weight-for-length table corresponding to the child's gender (Table I or Table II).
(ii) For children age 2 to attainment of age 18, we use the body mass index (BMI)-for-age table corresponding to the child's gender (Table III or Table IV).
(iii) BMI is the ratio of a child's weight to the square of his or her height. We calculate BMI using the formulas in 105.00G2c.
6. Complications of CKD. The hospitalizations in 106.09 may be for different complications of CKD. Examples of complications from CKD that may result in hospitalization include stroke, congestive heart failure, hypertensive crisis, or acute kidney failure requiring a short course of hemodialysis. If the CKD complication occurs during a hospitalization that was initially for a co-occurring condition, we will evaluate it under our rules for determining medical equivalence. (See § 416.926 of this chapter.) We will evaluate co-occurring conditions, including those that result in hospitalizations, under the listings for the affected body system or under our rules for medical equivalence.
D. How do we evaluate disorders that do not meet one of the genitourinary listings?1. The listed disorders are only examples of common genitourinary disorders that we consider severe enough to result in marked and severe functional limitations. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See § 416.926 of this chapter.) Genitourinary disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not medically equal a listing, we will also consider whether it functionally equals the listings. (See § 416.926a of this chapter.) We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
106.01 Category of Impairments, Genitourinary Disorders106.03 Chronic kidney disease, with chronic hemodialysis or peritoneal dialysis (see 106.00C1).
106.04 Chronic kidney disease, with kidney transplant. Consider under a disability for 1 year following the transplant; thereafter, evaluate the residual impairment (see 106.00C2).
106.05 Chronic kidney disease, with impairment of kidney function, with one of the following documented on at least two occasions at least 90 days apart during a consecutive 12-month period:
A. Serum creatinine of 3 mg/dL or greater;
ORB. Creatinine clearance of 30 ml/min/1.73m 2 or less;
ORC. Estimated glomerular filtration rate (eGFR) of 30 ml/min/1.73m 2 or less.
106.06 Nephrotic syndrome, with A and B:
A. Laboratory findings as described in 1 or 2, documented on at least two occasions at least 90 days apart during a consecutive 12-month period:
1. Serum albumin of 3.0 g/dL or less, or
2. Proteinuria of 40 mg/m 2/hr or greater;
ANDB. Anasarca (see 106.00C3) persisting for at least 90 days despite prescribed treatment.
106.07 Congenital genitourinary disorder (see 106.00C4) requiring urologic surgical procedures at least three times in a consecutive 12-month period, with at least 30 days between procedures. Consider under a disability for 1 year following the date of the last surgery; thereafter, evaluate the residual impairment.
106.09 Complications of chronic kidney disease (see 106.00C5) requiring at least three hospitalizations within a consecutive 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
106.08 Growth failure due to any chronic renal disease (see 106.00C5), with:
A. Serum creatinine of 2 mg/dL or greater, documented at least two times within a consecutive 12-month period with at least 60 days between measurements.
ANDB. Growth failure as required in 1 or 2:
1. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate weight-for-length table under 105.08B1; or
2. For children age 2 to attainment of age 18, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate BMI-for-age table under 105.08B2.
107.00 Hematological Disorders A. What hematological disorders do we evaluate under these listings?1. We evaluate non-malignant (non-cancerous) hematological disorders, such as hemolytic anemias (107.05), disorders of thrombosis and hemostasis (107.08), and disorders of bone marrow failure (107.10). These disorders disrupt the normal development and function of white blood cells, red blood cells, platelets, and clotting-factor proteins (factors).
2. We evaluate malignant (cancerous) hematological disorders, such as lymphoma, leukemia, and multiple myeloma, under the appropriate listings in 113.00, except for two lymphomas associated with human immunodeficiency virus (HIV) infection. We evaluate primary central nervous system lymphoma associated with HIV infection under 114.11B, and primary effusion lymphoma associated with HIV infection under 114.11C.
B. What evidence do we need to document that you have a hematological disorder?We need the following evidence to document that you have a hematological disorder:
1. A laboratory report of a definitive test that establishes a hematological disorder, signed by a physician; or
2. A laboratory report of a definitive test that establishes a hematological disorder that is not signed by a physician and a report from a physician that states you have the disorder; or
3. When we do not have a laboratory report of a definitive test, a persuasive report from a physician that a diagnosis of your hematological disorder was confirmed by appropriate laboratory analysis or other diagnostic method(s). To be persuasive, this report must state that you had the appropriate definitive laboratory test or tests for diagnosing your disorder and provide the results, or explain how your diagnosis was established by other diagnostic method(s) consistent with the prevailing state of medical knowledge and clinical practice.
4. We will make every reasonable effort to obtain the results of appropriate laboratory testing you have had. We will not purchase complex, costly, or invasive tests, such as tests of clotting-factor proteins, and bone marrow aspirations.
C. What are hemolytic anemias, and how do we evaluate them under 107.05?1. Hemolytic anemias, both congenital and acquired, are disorders that result in premature destruction of red blood cells (RBCs). Hemolytic anemias include abnormalities of hemoglobin structure (hemoglobinopathies), abnormal RBC enzyme content and function, and RBC membrane (envelope) defects that are congenital or acquired. The diagnosis of hemolytic anemia is based on hemoglobin electrophoresis or analysis of the contents of the RBC (enzymes) and membrane. Examples of congenital hemolytic anemias include sickle cell disease, thalassemia, and their variants, and hereditary spherocytosis. Acquired hemolytic anemias may result from autoimmune disease (for example, systemic lupus erythematosus) or mechanical devices (for example, heart valves, intravascular patches).
2. The hospitalizations in 107.05B do not all have to be for the same complication of the hemolytic anemia. They may be for three different complications of the disorder. Examples of complications of hemolytic anemia that may result in hospitalization include dactylitis, osteomyelitis, painful (vaso-occlusive) crisis, pulmonary infections or infarctions, acute chest syndrome, pulmonary hypertension, chronic heart failure, gallbladder disease, hepatic (liver) failure, renal (kidney) failure, nephrotic syndrome, aplastic crisis, and strokes. We will count the hours you receive emergency treatment in a comprehensive sickle cell disease center immediately before the hospitalization if this treatment is comparable to the treatment provided in a hospital emergency department.
3. For 107.05C, we do not require hemoglobin to be measured during a period in which you are free of pain or other symptoms of your disorder. We will accept hemoglobin measurements made while you are experiencing complications of your hemolytic anemia.
4. 107.05D refers to the most serious type of beta thalassemia major in which the bone marrow cannot produce sufficient numbers of normal RBCs to maintain life. The only available treatments for beta thalassemia major are life-long RBC transfusions (sometimes called hypertransfusion) or bone marrow transplantation. For purposes of 107.05D, we do not consider prophylactic RBC transfusions to prevent strokes or other complications in sickle cell disease and its variants to be of equal significance to life-saving RBC transfusions for beta thalassemia major. However, we will consider the functional limitations associated with prophylactic RBC transfusions and any associated side effects (for example, iron overload) under functional equivalence and any affected body system(s). We will also evaluate strokes and resulting complications under 111.00 and 112.00.
D. What are disorders of thrombosis and hemostasis, and how do we evaluate them under 107.08?1. Disorders of thrombosis and hemostasis include both clotting and bleeding disorders, and may be congenital or acquired. These disorders are characterized by abnormalities in blood clotting that result in hypercoagulation (excessive blood clotting) or hypocoagulation (inadequate blood clotting). The diagnosis of a thrombosis or hemostasis disorder is based on evaluation of plasma clotting-factor proteins (factors) and platelets. Protein C or protein S deficiency and Factor V Leiden are examples of hypercoagulation disorders. Hemophilia, von Willebrand disease, and thrombocytopenia are examples of hypocoagulation disorders. Acquired excessive blood clotting may result from blood protein defects and acquired inadequate blood clotting (for example, acquired hemophilia A) may be associated with inhibitor autoantibodies.
2. The hospitalizations in 107.08 do not all have to be for the same complication of a disorder of thrombosis and hemostasis. They may be for three different complications of the disorder. Examples of complications that may result in hospitalization include anemias, thromboses, embolisms, and uncontrolled bleeding requiring multiple factor concentrate infusions or platelet transfusions. We will also consider any surgery that you have, even if it is not related to your hematological disorder, to be a complication of your disorder of thrombosis and hemostasis if you require treatment with clotting-factor proteins (for example, factor VIII or IX) or anticoagulant medication to control bleeding or coagulation in connection with your surgery. We will count the hours you receive emergency treatment in a comprehensive hemophilia treatment center immediately before the hospitalization if this treatment is comparable to the treatment provided in a hospital emergency department.
E. What are disorders of bone marrow failure, and how do we evaluate them under 107.10?1. Disorders of bone marrow failure may be congenital or acquired, characterized by bone marrow that does not make enough healthy RBCs, platelets, or granulocytes (specialized types of white blood cells); there may also be a combined failure of these bone marrow-producing cells. The diagnosis is based on peripheral blood smears and bone marrow aspiration or bone marrow biopsy, but not peripheral blood smears alone. Examples of these disorders are myelodysplastic syndromes, aplastic anemia, granulocytopenia, and myelofibrosis. Acquired disorders of bone marrow failure may result from viral infections, chemical exposure, or immunologic disorders.
2. The hospitalizations in 107.10A do not all have to be for the same complication of bone marrow failure. They may be for three different complications of the disorder. Examples of complications that may result in hospitalization include uncontrolled bleeding, anemia, and systemic bacterial, viral, or fungal infections.
3. For 107.10B, the requirement of life-long RBC transfusions to maintain life in myelodysplastic syndromes or aplastic anemias has the same meaning as it does for beta thalassemia major. (See 107.00C4.)
F. How do we evaluate bone marrow or stem cell transplantation under 107.17?We will consider you to be disabled for 12 months from the date of bone marrow or stem cell transplantation, or we may consider you to be disabled for a longer period if you are experiencing any serious post-transplantation complications, such as graft-versus-host (GVH) disease, frequent infections after immunosuppressive therapy, or significant deterioration of organ systems. We do not restrict our determination of the onset of disability to the date of the transplantation in 107.17. We may establish an earlier onset of disability due to your transplantation if evidence in your case record supports such a finding.
G. How do we consider your symptoms, including your pain, severe fatigue, and malaise?Your symptoms, including pain, severe fatigue, and malaise, may be important factors in our determination whether your hematological disorder meets or medically equals a listing, or in our determination whether you otherwise have marked and severe functional limitations. We cannot consider your symptoms unless you have medical signs or laboratory findings showing the existence of a medically determinable impairment(s) that could reasonably be expected to produce the symptoms. If you have such an impairment(s), we will evaluate the intensity, persistence, and functional effects of your symptoms using the rules throughout 107.00 and in our other regulations. (See sections 416.921 and 416.929 of this chapter.) Additionally, when we assess the credibility of your complaints about your symptoms and their functional effects, we will not draw any inferences from the fact that you do not receive treatment or that you are not following treatment without considering all of the relevant evidence in your case record, including any explanations you provide on why you are not receiving or following treatment.
H. How do we evaluate episodic events in hematological disorders?Some of the listings in this body system require a specific number of events within a consecutive 12-month period. (See 107.05, 107.08, and 107.10A.) When we use such criteria, a consecutive 12-month period means a period of 12 consecutive months, all or part of which must occur within the period we are considering in connection with your application or continuing disability review. These events must occur at least 30 days apart to ensure that we are evaluating separate events.
I. How do we evaluate hematological disorders that do not meet one of these listings?1. These listings are only common examples of hematological disorders that we consider severe enough to result in marked and severe functional limitations. If your disorder does not meet the criteria of any of these listings, we must consider whether you have a disorder that satisfies the criteria of a listing in another body system. For example, we will evaluate hemophilic joint deformity under 101.00; polycythemia vera under 103.00, 104.00, or 111.00; chronic iron overload resulting from repeated RBC transfusion (transfusion hemosiderosis) under 103.00, 104.00, or 105.00; and the effects of intracranial bleeding or stroke under 111.00 or 112.00.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See section 416.926 of this chapter.) Hematological disorders may be associated with disorders in other body systems, and we consider the combined effects of multiple impairments when we determine whether they medically equal a listing. If your impairment(s) does not medically equal a listing, we will also consider whether it functionally equals the listings. (See section 416.926a of this chapter.) We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
107.01 Category of Impairments, Hematological Disorders
107.05 Hemolytic anemias, including sickle cell disease, thalassemia, and their variants (see 107.00C), with:
A. Documented painful (vaso-occlusive) crises requiring parenteral (intravenous or intramuscular) narcotic medication, occurring at least six times within a 12-month period with at least 30 days between crises.
OR
B. Complications of hemolytic anemia requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department or comprehensive sickle cell disease center immediately before the hospitalization (see 107.00C2).
OR
C. Hemoglobin measurements of 7.0 grams per deciliter (g/dL) or less, occurring at least three times within a 12-month period with at least 30 days between measurements.
OR
D. Beta thalassemia major requiring life-long RBC transfusions at least once every 6 weeks to maintain life (see 107.00C4).
107.08 Disorders of thrombosis and hemostasis, including hemophilia and thrombocytopenia (see 107.00D), with complications requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department or comprehensive hemophilia treatment center immediately before the hospitalization (see 107.00D2).
107.10 Disorders of bone marrow failure, including myelodysplastic syndromes, aplastic anemia, granulocytopenia, and myelofibrosis (see 107.00E), with:
A. Complications of bone marrow failure requiring at least three hospitalizations within a 12-month period and occurring at least 30 days apart. Each hospitalization must last at least 48 hours, which can include hours in a hospital emergency department immediately before the hospitalization (see 107.00E2).
OR
B. Myelodysplastic syndromes or aplastic anemias requiring life-long RBC transfusions at least once every 6 weeks to maintain life (see 107.00E3).
107.17 Hematological disorders treated by bone marrow or stem cell transplantation (see 107.00F). Consider under a disability for at least 12 consecutive months from the date of transplantation. After that, evaluate any residual impairment(s) under the criteria for the affected body system.
108.00 Skin DisordersA. What skin disorders do we evaluate with these listings? We use these listings to evaluate skin disorders that may result from hereditary, congenital, or acquired pathological processes. The kinds of impairments covered by these listings are: Ichthyosis, bullous diseases, chronic infections of the skin or mucous membranes, dermatitis, hidradenitis suppurativa, genetic photosensitivity disorders, and burns.
B. What documentation do we need? When we evaluate the existence and severity of your skin disorder, we generally need information about the onset, duration, frequency of flareups, and prognosis of your skin disorder; the location, size, and appearance of lesions; and, when applicable, history of exposure to toxins, allergens, or irritants, familial incidence, seasonal variation, stress factors, and your ability to function outside of a highly protective environment. To confirm the diagnosis, we may need laboratory findings (for example, results of a biopsy obtained independently of Social Security disability evaluation or blood tests) or evidence from other medically acceptable methods consistent with the prevailing state of medical knowledge and clinical practice.
C. How do we assess the severity of your skin disorders(s)? We generally base our assessment of severity on the extent of your skin lesions, the frequency of flareups of your skin lesions, how your symptoms (including pain) limit you, the extent of your treatment, and how your treatment affects you.
1. Extensive skin lesions. Extensive skin lesions are those that involve multiple body sites or critical body areas, and result in a very serious limitation. Examples of extensive skin lesions that result in a very serious limitation include but are not limited to:
a. Skin lesions that interfere with the motion of your joints and that very seriously limit your use of more than one extremity; that is, two upper extremities, two lower extremities, or one upper and one lower extremity.
b. Skin lesions on the palms of both hands that very seriously limit your ability to do fine and gross motor movements.
c. Skin lesions on the soles of both feet, the perineum, or both inguinal areas that very seriously limit your ability to ambulate.
2. Frequency of flareups. If you have skin lesions, but they do not meet the requirements of any of the listings in this body system, you may still have an impairment that results in marked and severe functional limitations when we consider your condition over time, especially if your flareups result in extensive skin lesions, as defined in C1 of this section. Therefore, if you have frequent flareups, we may find that your impairment(s) is medically equal to one of these listings even though you have some periods during which your condition is in remission. We will consider how frequent and serious your flareups are, how quickly they resolve, and how you function between flareups to determine whether you have marked and severe functional limitations that have lasted for a continuous period of at least 12 months or that can be expected to last for a continuous period of at least 12 months. We will also consider the frequency of your flareups when we determine whether you have a severe impairment and when we need to assess functional equivalence.
3. Symptoms (including pain). Symptoms (including pain) may be important factors contributing to the severity of your skin disorder(s). We assess the impact of symptoms as explained in §§ 416.921 and 416.929 of this chapter.
4. Treatment. We assess the effects of medication, therapy, surgery, and any other form of treatment you receive when we determine the severity and duration of your impairment(s). Skin disorders frequently respond to treatment; however, response to treatment can vary widely, with some impairments becoming resistant to treatment. Some treatments can have side effects that can in themselves result in limitations.
a. We assess the effects of continuing treatment as prescribed by determining if there is improvement in the symptoms, signs, and laboratory findings of your disorder, and if you experience side effects that result in functional limitations. To assess the effects of your treatment, we may need information about:
i. The treatment you have been prescribed (for example, the type, dosage, method and frequency of administration of medication or therapy);
ii. Your response to the treatment;
iii. Any adverse effects of the treatment; and
iv. The expected duration of the treatment.
b. Because treatment itself or the effects of treatment may be temporary, in most cases sufficient time must elapse to allow us to evaluate the impact and expected duration of treatment and its side effects. Except under 108.07 and 108.08, you must follow continuing treatment as prescribed for at least 3 months before your impairment can be determined to meet the requirements of a skin disorder listing. (See 108.00H if you are not undergoing treatment or did not have treatment for 3 months.) We consider your specific response to treatment when we evaluate the overall severity of your impairment.
D. How do we assess impairments that may affect the skin and other body systems? When your impairment affects your skin and has effects in other body systems, we first evaluate the predominant feature of your impairment under the appropriate body system. Examples include, but are not limited to the following.
1. Tuberous sclerosis primarily affects the brain. The predominant features are seizures, which we evaluate under the neurological listings in 111.00, and developmental delays or other mental disorders, which we evaluate under the mental disorders listings in 112.00.
2. Malignant tumors of the skin (for example, malignant melanoma) are cancers, or neoplastic diseases, which we evaluate under the listings in 113.00.
3. Autoimmune disorders and other immune system disorders (for example, systemic lupus erythematosus (SLE), scleroderma, human immunodeficiency virus (HIV) infection, and Sjögren's syndrome) often involve more than one body system. We first evaluate these disorders under the immune system disorders listings in 114.00. We evaluate SLE under 114.02, scleroderma under 114.04, Sjögren's syndrome under 114.10, and HIV infection under 114.11.
4. Disfigurement or deformity resulting from skin lesions may result in loss of sight, hearing, speech, and the ability to chew (mastication). We evaluate these impairments and their effects under the special senses and speech listings in 102.00 and the digestive system listings in 105.00. Facial disfigurement or other physical deformities may also have effects we evaluate under the mental disorders listings in 112.00, such as when they affect mood or social functioning.
5. We evaluate erythropoietic porphyrias under the hemic and lymphatic listings in 107.00.
6. We evaluate hemangiomas associated with thrombocytopenia and hemorrhage (for example, Kasabach-Merritt syndrome) involving coagulation defects, under the hemic and lymphatic listings in 107.00. But, when hemangiomas impinge on vital structures or interfere with function, we evaluate their primary effects under the appropriate body system.
E. How do we evaluate genetic photosensitivity disorders?
1. Xeroderma pigmentosum (XP). When you have XP, your impairment meets the requirements of 108.07A if you have clinical and laboratory findings showing that you have the disorder. (See 108.00E3.) People who have XP have a lifelong hypersensitivity to all forms of ultraviolet light and generally lead extremely restricted lives in highly protective environments in order to prevent skin cancers from developing. Some people with XP also experience problems with their eyes, neurological problems, mental disorders, and problems in other body systems.
2. Other genetic photosensitivity disorders. Other genetic photosensitivity disorders may vary in their effects on different people, and may not result in marked and severe functional limitations for a continuous period of at least 12 months. Therefore, if you have a genetic photosensitivity disorder other than XP (established by clinical and laboratory findings as described in 108.00E3), you must show that you have either extensive skin lesions or an inability to function outside of a highly protective environment to meet the requirements of 108.07B. You must also show that your impairment meets the duration requirement. By inability to function outside of a highly protective environment we mean that you must avoid exposure to ultraviolet light (including sunlight passing through windows and light from unshielded fluorescent bulbs), wear protective clothing and eyeglasses, and use opaque broad-spectrum sunscreens in order to avoid skin cancer or other serious effects. Some genetic photosensitivity disorders can have very serious effects in other body systems, especially special senses and speech (102.00), neurological (111.00), mental (112.00), and neoplastic (113.00). We will evaluate the predominant feature of your impairment under the appropriate body system, as explained in 108.00D.
3. Clinical and laboratory findings.
a. General. We need documentation from an acceptable medical source to establish that you have a medically determinable impairment. In general, we must have evidence of appropriate laboratory testing showing that you have XP or another genetic photosensitivity disorder. We will find that you have XP or another genetic photosensitivity disorder based on a report from an acceptable medical source indicating that you have the impairment, supported by definitive genetic laboratory studies documenting appropriate chromosomal changes, including abnormal DNA repair or another DNA or genetic abnormality specific to your type of photosensitivity disorder.
b. What we will accept as medical evidence instead of the actual laboratory report. When we do not have the actual laboratory report, we need evidence from an acceptable medical source that includes appropriate clinical findings for your impairment and that is persuasive that a positive diagnosis has been confirmed by appropriate laboratory testing at some time prior to our evaluation. To be persuasive, the report must state that the appropriate definitive genetic laboratory study was conducted and that the results confirmed the diagnosis. The report must be consistent with other evidence in your case record.
F. How do we evaluate burns? Electrical, chemical, or thermal burns frequently affect other body systems; for example, musculoskeletal, special senses and speech, respiratory, cardiovascular, renal, neurological, or mental. Consequently, we evaluate burns the way we evaluate other disorders that can affect the skin and other body systems, using the listing for the predominant feature of your impairment. For example, if your soft tissue injuries are under continuing surgical management (as defined in 101.00M), we will evaluate your impairment under 101.08. However, if your burns do not meet the requirements of 101.08 and you have extensive skin lesions that result in a very serious limitation (as defined in 108.00C1) that has lasted or can be expected to last for a continuous period of at least 12 months, we will evaluate them under 108.08.
G. How do we determine if your skin disorder(s) will continue at a disabling level of severity in order to meet the duration requirement? For all of these skin disorder listings except 108.07 and 108.08, we will find that your impairment meets the duration requirement if your skin disorder results in extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed. By persist, we mean that the longitudinal clinical record shows that, with few exceptions, your lesions have been at the level of severity specified in the listing. For 108.07A, we will presume that you meet the duration requirement. For 108.07B and 108.08, we will consider all of the relevant medical and other information in your case record to determine whether your skin disorder meets the duration requirement.
H. How do we assess your skin disorder(s) if your impairment does not meet the requirements of one of these listings?
1. These listings are only examples of common skin disorders that we consider severe enough to result in marked and severe functional limitations. For most of these listings, if you do not have continuing treatment as prescribed, if your treatment has not lasted for at least 3 months, or if you do not have extensive skin lesions that have persisted for at least 3 months, your impairment cannot meet the requirements of these skin disorder listings. (This provision does not apply to 108.07 and 108.08.) However, we may still find that you are disabled because your impairment(s) meets the requirements of a listing in another body system, medically equals (see §§ 404.1526 and 416.926 of this chapter) the severity of a listing, or functionally equals the severity of the listings.
2. If you have not received ongoing treatment or do not have an ongoing relationship with the medical community despite the existence of a severe impairment(s), or if your skin lesions have not persisted for at least 3 months but you are undergoing continuing treatment as prescribed, you may still have an impairment(s) that meets a listing in another body system or that medically equals a listing. If you do not have an impairment(s) that meets or medically equals a listing, we will consider whether your impairment(s) functionally equals the listings. (See § 416.924 of this chapter.) When we decide whether you continue to be disabled, we use the rules in § 416.994a of this chapter.
108.01 Category of Impairments, Skin Disorders108.02 Ichthyosis, with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
108.03 Bullous disease (for example, pemphigus, erythema multiforme bullosum, epidermolysis bullosa, bullous pemphigoid, dermatitis herpetiformis), with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
108.04 Chronic infections of the skin or mucous membranes, with extensive fungating or extensive ulcerating skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
108.05 Dermatitis (for example, psoriasis, dyshidrosis, atopic dermatitis, exfoliative dermatitis, allergic contact dermatitis), with extensive skin lesions that persist for at least 3 months despite continuing treatment as prescribed.
108.06 Hidradenitis suppurativa, with extensive skin lesions involving both axillae, both inguinal areas, or the perineum that persist for at least 3 months despite continuing treatment as prescribed.
108.07 Genetic photosensitivity disorders, established as described in 108.00E.
A. Xeroderma pigmentosum. Consider the individual disabled from birth.
B. Other genetic photosensitivity disorders, with:
1. Extensive skin lesions that have lasted or can be expected to last for a continuous period of at least 12 months, or
2. Inability to function outside of a highly protective environment for a continuous period of at least 12 months (see 108.00E2).
108.08 Burns, with extensive skin lesions that have lasted or can be expected to last for a continuous period of at least 12 months. (See 108.00F).
109.00 Endocrine DisordersA. What is an endocrine disorder?
An endocrine disorder is a medical condition that causes a hormonal imbalance. When an endocrine gland functions abnormally, producing either too much of a specific hormone (hyperfunction) or too little (hypofunction), the hormonal imbalance can cause various complications in the body. The major glands of the endocrine system are the pituitary, thyroid, parathyroid, adrenal, and pancreas.
B. How do we evaluate the effects of endocrine disorders? The only listing in this body system addresses children from birth to the attainment of age 6 who have diabetes mellitus (DM) and require daily insulin. We evaluate other impairments that result from endocrine disorders under the listings for other body systems. For example:
1. Pituitary gland disorders can disrupt hormone production and normal functioning in other endocrine glands and in many body systems. The effects of pituitary gland disorders vary depending on which hormones are involved. For example, when pituitary growth hormone deficiency in growing children limits bone maturation and results in pathological short stature, we evaluate this linear growth impairment under 100.00. When pituitary hypofunction affects water and electrolyte balance in the kidney and leads to diabetes insipidus, we evaluate the effects of recurrent dehydration under 106.00.
2. Thyroid gland disorders affect the sympathetic nervous system and normal metabolism. We evaluate thyroid-related changes in linear growth under 100.00; thyroid-related changes in blood pressure and heart rate that cause cardiac arrhythmias or other cardiac dysfunction under 104.00; thyroid-related weight loss under 105.00; and cognitive limitations, mood disorders, and anxiety under 112.00.
3. Parathyroid gland disorders affect calcium levels in bone, blood, nerves, muscle, and other body tissues. We evaluate parathyroid-related osteoporosis and fractures under 101.00; abnormally elevated calcium levels in the blood (hypercalcemia) that lead to cataracts under 102.00; kidney failure under 106.00; and recurrent abnormally low blood calcium levels (hypocalcemia) that lead to increased excitability of nerves and muscles, such as tetany and muscle spasms, under 111.00.
4. Adrenal gland disorders affect bone calcium levels, blood pressure, metabolism, and mental status. We evaluate adrenal-related linear growth impairments under 100.00; adrenal-related osteoporosis with fractures that compromises the ability to walk or to use the upper extremities under 101.00; adrenal-related hypertension that worsens heart failure or causes recurrent arrhythmias under 104.00; adrenal-related weight loss under 105.00; and mood disorders under 112.00.
5. Diabetes mellitus and other pancreatic gland disorders disrupt the production of several hormones, including insulin, that regulate metabolism and digestion. Insulin is essential to the absorption of glucose from the bloodstream into body cells for conversion into cellular energy. The most common pancreatic gland disorder is diabetes mellitus (DM). There are two major types of DM: type 1 and type 2. Both type 1 and type 2 DM are chronic disorders that can have serious, disabling complications that meet the duration requirement. Type 1 DM - previously known as “juvenile diabetes” or “insulin-dependent diabetes mellitus” (IDDM) - is an absolute deficiency of insulin secretion that commonly begins in childhood and continues throughout adulthood. Treatment of type 1 DM always requires lifelong daily insulin. With type 2 DM - previously known as “adult-onset diabetes mellitus” or “non-insulin-dependent diabetes mellitus” (NIDDM) - the body's cells resist the effects of insulin, impairing glucose absorption and metabolism. Type 2 is less common than type 1 DM in children, but physicians are increasingly diagnosing type 2 DM before age 18. Treatment of type 2 DM generally requires lifestyle changes, such as increased exercise and dietary modification, and sometimes insulin in addition to other medications. While both type 1 and type 2 DM are usually controlled, some children do not achieve good control for a variety of reasons including, but not limited to, hypoglycemia unawareness, other disorders that can affect blood glucose levels, inability to manage DM due to a mental disorder, or inadequate treatment.
a. Hyperglycemia. Both types of DM cause hyperglycemia, which is an abnormally high level of blood glucose that may produce acute and long-term complications. Acute complications of hyperglycemia include diabetic ketoacidosis. Long-term complications of chronic hyperglycemia include many conditions affecting various body systems but are rare in children.
b. Diabetic ketoacidosis (DKA). DKA is an acute, potentially life-threatening complication of DM in which the chemical balance of the body becomes dangerously hyperglycemic and acidic. It results from a severe insulin deficiency, which can occur due to missed or inadequate daily insulin therapy or in association with an acute illness. It usually requires hospital treatment to correct the acute complications of dehydration, electrolyte imbalance, and insulin deficiency. You may have serious complications resulting from your treatment, which we evaluate under the affected body system. For example, we evaluate cardiac arrhythmias under 104.00, intestinal necrosis under 105.00, and cerebral edema and seizures under 111.00. Recurrent episodes of DKA in adolescents may result from mood or eating disorders, which we evaluate under 112.00.
c. Hypoglycemia. Children with DM may experience episodes of hypoglycemia, which is an abnormally low level of blood glucose. Most children age 6 and older recognize the symptoms of hypoglycemia and reverse them by consuming substances containing glucose; however, some do not take this step because of hypoglycemia unawareness. Severe hypoglycemia can lead to complications, including seizures or loss of consciousness, which we evaluate under 111.00, or altered mental status, cognitive deficits, and permanent brain damage, which we evaluate under 112.00.
C. How do we evaluate DM in children?
Listing 109.08 is only for children with DM who have not attained age 6 and who require daily insulin. For all other children (that is, children with DM who are age 6 or older and require daily insulin, and children of any age with DM who do not require daily insulin), we follow our rules for determining whether the DM is severe, alone or in combination with another impairment, whether it meets or medically equals the criteria of a listing in another body system, or functionally equals the listings under the criteria in § 416.926a, considering the factors in § 416.924a. The management of DM in children can be complex and variable from day to day, and all children with DM require some level of adult supervision. For example, if a child age 6 or older has a medical need for 24-hour-a-day adult supervision of insulin treatment, food intake, and physical activity to ensure survival, we will find that the child's impairment functionally equals the listings based on the example in § 416.926a(m)(5).
D. How do we evaluate other endocrine disorders that do not have effects that meet or medically equal the criteria of any listing in other body systems? If your impairment(s) does not meet or medically equal a listing in another body system, we will consider whether your impairment(s) functionally equals the listings under the criteria in § 416.926a, considering the factors in § 416.924a. When we decide whether you continue to be disabled, we use the rules in § 416.994a.
109.01 Category of Impairments, Endocrine
109.08 Any type of diabetes mellitus in a child who requires daily insulin and has not attained age 6. Consider under a disability until the attainment of age 6. Thereafter, evaluate the diabetes mellitus according to the rules in 109.00B5 and C.
110.00 Congenital Disorders That Affect Multiple Body SystemsA. Which disorders do we evaluate under this body system? We evaluate non-mosaic Down syndrome and catastrophic congenital disorders under this body system.
B. What is non-mosaic Down syndrome? Non-mosaic Down syndrome is a genetic disorder. Most children with non-mosaic Down syndrome have three copies of chromosome 21 in all of their cells (chromosome 21 trisomy); some have an extra copy of chromosome 21 attached to a different chromosome in all of their cells (chromosome 21 translocation). Virtually all children with non-mosaic Down syndrome have characteristic facial or other physical features, delayed physical development, and intellectual disability. Children with non-mosaic Down syndrome may also have congenital heart disease, impaired vision, hearing problems, and other disorders. We evaluate non-mosaic Down syndrome under 110.06. If you have non-mosaic Down syndrome documented as described in 110.00C, we consider you disabled from birth.
C. What evidence do we need to document non-mosaic Down syndrome under 110.06?1. Under 110.06A, we will find you disabled based on laboratory findings.
a. To find that your disorder meets 110.06A, we need a copy of the laboratory report of karyotype analysis, which is the definitive test to establish non-mosaic Down syndrome. We will not purchase karyotype analysis. We will not accept a fluorescence in situ hybridization (FISH) test because it does not distinguish between the mosaic and non-mosaic forms of Down syndrome.
b. If a physician (see §§ 404.1513(a)(1) and 416.913(a)(1) of this chapter) has not signed the laboratory report of karyotype analysis, the evidence must also include a physician's statement that you have Down syndrome.
c. For purposes of 110.06A, we do not require evidence stating that you have the distinctive facial or other physical features of Down syndrome.
2. If we do not have a laboratory report of karyotype analysis documenting that you have non-mosaic Down syndrome, we may find you disabled under 110.06B or 110.06C.
a. Under 110.06B, we need a physician's report stating: (i) your karyotype diagnosis or evidence that documents your type of Down syndrome that is consistent with prior karyotype analysis (for example, reference to a diagnosis of “trisomy 21”) and (ii) that you have the distinctive facial or other physical features of Down syndrome. We do not require a detailed description of the facial or other physical features of the disorder. However, we will not find that your disorder meets 110.06B if we have evidence - such as evidence of functioning inconsistent with the diagnosis - that indicates that you do not have non-mosaic Down syndrome.
b. If we do not have evidence of prior karyotype analysis (you did not have testing, or you had testing but we do not have information from a physician about the test results), we will find that your disorder meets 110.06C if we have: (i) a physician's report stating that you have the distinctive facial or other physical features of Down syndrome and (ii) evidence that your functioning is consistent with a diagnosis of non-mosaic Down syndrome. This evidence may include medical or nonmedical information about your physical and mental abilities, including information about your development, education, work history, or the results of psychological testing. However, we will not find that your disorder meets 110.06C if we have evidence - such as evidence of functioning inconsistent with the diagnosis - that indicates that you do not have non-mosaic Down syndrome.
D. What are catastrophic congenital disorders? Some catastrophic congenital disorders, such as anencephaly, cyclopia, chromosome 13 trisomy (Patau syndrome or trisomy D), and chromosome 18 trisomy (Edwards' syndrome or trisomy E), are usually expected to result in early death. Others such as cri du chat syndrome (chromosome 5p deletion syndrome) and the infantile onset form of Tay-Sachs disease interfere very seriously with development. We evaluate catastrophic congenital disorders under 110.08. The term “very seriously” in 110.08 has the same meaning as in the term “extreme” in § 416.926a(e)(3) of this chapter.
E. What evidence do we need under 110.08?
We need one of the following to determine if your disorder meets 110.08A or B:
1. A laboratory report of the definitive test that documents your disorder (for example, genetic analysis or evidence of biochemical abnormalities) signed by a physician.
2. A laboratory report of the definitive test that documents your disorder that is not signed by a physician and a report from a physician stating that you have the disorder.
3. A report from a physician stating that you have the disorder with the typical clinical features of the disorder and that you had definitive testing that documented your disorder. In this case, we will find that your disorder meets 110.08A or B unless we have evidence that indicates that you do not have the disorder.
4. If we do not have the definitive laboratory evidence we need under E1, E2, or E3, we will find that your disorder meets 110.08A or B if we have: (i) a report from a physician stating that you have the disorder and that you have the typical clinical features of the disorder, and (ii) other evidence that supports the diagnosis. This evidence may include medical or nonmedical information about your development and functioning.
5. For obvious catastrophic congenital anomalies that are expected to result in early death, such as anencephaly and cyclopia, we need evidence from a physician that demonstrates that the infant has the characteristic physical features of the disorder. In these rare cases, we do not need laboratory testing or any other evidence that confirms the disorder.
F. How do we evaluate mosaic Down syndrome and other congenital disorders that affect multiple body systems?
1. Mosaic Down syndrome. Approximately 2 percent of children with Down syndrome have the mosaic form. In mosaic Down syndrome, there are some cells with an extra copy of chromosome 21 and other cells with the normal two copies of chromosome 21. Mosaic Down syndrome can be so slight as to be undetected clinically, but it can also be profound and disabling, affecting various body systems.
2. Other congenital disorders that affect multiple body systems. Other congenital disorders, such as congenital anomalies, chromosomal disorders, dysmorphic syndromes, inborn metabolic syndromes, and perinatal infectious diseases, can cause deviation from, or interruption of, the normal function of the body or can interfere with development. Examples of these disorders include both the juvenile and late-onset forms of Tay-Sachs disease, trisomy X syndrome (XXX syndrome), fragile X syndrome, phenylketonuria (PKU), caudal regression syndrome, and fetal alcohol syndrome. For these disorders and other disorders like them, the degree of deviation, interruption, or interference, as well as the resulting functional limitations and their progression, may vary widely from child to child and may affect different body systems.
3. Evaluating the effects of mosaic Down syndrome or another congenital disorder under the listings. When the effects of mosaic Down syndrome or another congenital disorder that affects multiple body systems are sufficiently severe we evaluate the disorder under the appropriate affected body system(s), such as musculoskeletal, special senses and speech, neurological, or mental disorders. Otherwise, we evaluate the specific functional limitations that result from the disorder under our other rules described in 110.00G.
G. What if your disorder does not meet a listing? If you have a severe medically determinable impairment(s) that does not meet a listing, we will consider whether your impairment(s) medically equals a listing. See § 416.926 of this chapter. If your impairment(s) does not meet or medically equal a listing, we will consider whether it functionally equals the listings. See §§ 416.924a and 416.926a of this chapter. We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
110.01 Category of Impairments, Congenital Disorders That Affect Multiple Body Systems110.06 Non-mosaic Down syndrome (chromosome 21 trisomy or chromosome 21 translocation), documented by:
A. A laboratory report of karyotype analysis signed by a physician, or both a laboratory report of karyotype analysis not signed by a physician and a statement by a physician that the child has Down syndrome (see 110.00C1), or
B. A physician's report stating that the child has chromosome 21 trisomy or chromosome 21 translocation consistent with karyotype analysis with the distinctive facial or other physical features of Down syndrome (see 110.00C2a), or
C. A physician's report stating that the child has Down syndrome with the distinctive facial or other physical features and evidence demonstrating that the child is functioning at the level of a child with non-mosaic Down syndrome (see 110.00C2b).
110.08 A catastrophic congenital disorder (see 110.00D and 110.00E) with:
A. Death usually expected within the first months of life, or
B. Very serious interference with development or functioning.
111.00 Neurological DisordersA. Which neurological disorders do we evaluate under these listings? We evaluate epilepsy, coma or persistent vegetative state (PVS), and neurological disorders that cause disorganization of motor function, bulbar and neuromuscular dysfunction, or communication impairment. Under this body system, we evaluate the limitations resulting from the impact of the neurological disease process itself. If you have a neurological disorder(s) that affects your physical and mental functioning, we will evaluate your impairments under the rules we use to determine functional equivalence. If your neurological disorder results in only mental impairment or if you have a co-occurring mental condition that is not caused by your neurological disorder (for example, Autism spectrum disorder), we will evaluate your mental impairment under the mental disorders body system, 112.00.
B. What evidence do we need to document your neurological disorder?
1. We need both medical and non-medical evidence (signs, symptoms, and laboratory findings) to assess the effects of your neurological disorder. Medical evidence should include your medical history, examination findings, relevant laboratory tests, and the results of imaging. Imaging refers to medical imaging techniques, such as x-ray, computerized tomography (CT), magnetic resonance imaging (MRI), and electroencephalography (EEG). The imaging must be consistent with the prevailing state of medical knowledge and clinical practice as the proper technique to support the evaluation of the disorder. In addition, the medical evidence may include descriptions of any prescribed treatment and your response to it. We consider non-medical evidence such as statements you or others make about your impairments, your restrictions, your daily activities, or, if you are an adolescent, your efforts to work.
2. We will make every reasonable effort to obtain the results of your laboratory and imaging evidence. When the results of any of these tests are part of the existing evidence in your case record, we will evaluate the test results and all other relevant evidence. We will not purchase imaging, or other diagnostic tests or laboratory tests that are complex, may involve significant risk, or that are invasive. We will not routinely purchase tests that are expensive or not readily available.
C. How do we consider adherence to prescribed treatment in neurological disorders? In 111.02 (Epilepsy) and 111.12 (Myasthenia gravis), we require that limitations from these neurological disorders exist despite adherence to prescribed treatment. “Despite adherence to prescribed treatment” means that you have taken medication(s) or followed other treatment procedures for your neurological disorder(s) as prescribed by a physician for three consecutive months but your impairment continues to meet the other listing requirements despite this treatment. You may receive your treatment at a health care facility that you visit regularly, even if you do not see the same physician on each visit.
D. What do we mean by disorganization of motor function?
1. Disorganization of motor function means interference, due to your neurological disorder, with movement of two extremities; i.e., the lower extremities, or upper extremities (including fingers, wrists, hands, arms, and shoulders). By two extremities we mean both lower extremities, or both upper extremities, or one upper extremity and one lower extremity. All listings in this body system, except for 111.02 (Epilepsy) and 111.20 (Coma and persistent vegetative state), include criteria for disorganization of motor function that results in an extreme limitation in your ability to:
a. Stand up from a seated position; or
b. Balance while standing or walking; or
c. Use the upper extremities (e.g., fingers, wrists, hands, arms, and shoulders).
2. Extreme limitation means the inability to stand up from a seated position, maintain balance in a standing position and while walking, or use your upper extremities to independently initiate, sustain, and complete age-appropriate activities. The assessment of motor function depends on the degree of interference with standing up; balancing while standing or walking; or using the upper extremities (including fingers, hands, arms, and shoulders).
a. Inability to stand up from a seated position means that once seated you are unable to stand and maintain an upright position without the assistance of another person or the use of an assistive device, such as a walker, two crutches, or two canes.
b. Inability to maintain balance in a standing position means that you are unable to maintain an upright position while standing or walking without the assistance of another person or an assistive device, such as a walker, two crutches, or two canes.
c. Inability to use your upper extremities means that you have a loss of function of both upper extremities (e.g., fingers, wrists, hands, arms, and shoulders) that very seriously limits your ability to independently initiate, sustain, and complete age- appropriate activities involving fine and gross motor movements. Inability to perform fine and gross motor movements could include not being able to pinch, manipulate, and use your fingers; or not being able to use your hands, arms, and shoulders to perform gross motor movements, such as handling, gripping, grasping, holding, turning, and reaching; or not being able to engage in exertional movements such a lifting, carrying, pushing, and pulling.
3. For children who are not yet able to balance, stand up, or walk independently, we consider their function based on assessments of limitations in the ability to perform comparable age-appropriate activities with the lower and upper extremities, given normal developmental milestones. For such children, an extreme level of limitation means developmental milestones at less than one-half of the child's chronological age.
E. What do we mean by bulbar and neuromuscular dysfunction? The bulbar region of the brain is responsible for controlling the bulbar muscles in the throat, tongue, jaw, and face. Bulbar and neuromuscular dysfunction refers to weakness in these muscles, resulting in breathing, swallowing, and speaking impairments. Listings 111.12 (Myasthenia gravis) and 111.22 (Motor neuron disorders) include criteria for evaluating bulbar and neuromuscular dysfunction. If your neurological disorder has resulted in a breathing disorder, we may evaluate that condition under the respiratory system, 103.00.
F. What is epilepsy, and how do we evaluate it under 111.02?
1. Epilepsy is a pattern of recurrent and unprovoked seizures that are manifestations of abnormal electrical activity in the brain. There are various types of generalized and “focal” or partial seizures. In children, the most common potentially disabling seizure types are generalized tonic-clonic seizures, dyscognitive seizures (formerly complex partial seizures), and absence seizures. However, psychogenic nonepileptic seizures and pseudoseizures are not epileptic seizures for the purpose of 111.02. We evaluate psychogenic seizures and pseudoseizures under the mental disorders body system, 112.00.
a. Generalized tonic-clonic seizures are characterized by loss of consciousness accompanied by a tonic phase (sudden muscle tensing causing the child to lose postural control) followed by a clonic phase (rapid cycles of muscle contraction and relaxation, also called convulsions). Tongue biting and incontinence may occur during generalized tonic-clonic seizures, and injuries may result from falling.
b. Dyscognitive seizures are characterized by alteration of consciousness without convulsions or loss of muscle control. During the seizure, blank staring, change of facial expression, and automatisms (such as lip smacking, chewing or swallowing, or repetitive simple actions, such as gestures or verbal utterances) may occur. During its course, a dyscognitive seizure may progress into a generalized tonic-clonic seizure (see 111.00F1a).
c. Absence seizures (petit mal) are also characterized by an alteration in consciousness, but are shorter than other generalized seizures (e.g., tonic-clonic and dyscognitive) seizures, generally lasting for only a few seconds rather than minutes. They may present with blank staring, change of facial expression, lack of awareness and responsiveness, and a sense of lost time after the seizure. An aura never precedes absence seizures. Although absence seizures are brief, frequent occurrence may limit functioning. This type of seizure usually does not occur after adolescence.
d. Febrile seizures may occur in young children in association with febrile illnesses. We will consider seizures occurring during febrile illnesses. To meet 111.02, we require documentation of seizures during nonfebrile periods and epilepsy must be established.
2. Description of seizure. We require at least one detailed description of your seizures from someone, preferably a medical professional, who has observed at least one of your typical seizures. If you experience more than one type of seizure, we require a description of each type.
3. Serum drug levels. We do not require serum drug levels; therefore, we will not purchase them. However, if serum drug levels are available in your medical records, we will evaluate them in the context of the other evidence in your case record.
4. Counting seizures. The period specified in 111.02A or B cannot begin earlier than one month after you began prescribed treatment. The required number of seizures must occur within the period we are considering in connection with your application or continuing disability review. When we evaluate the frequency of your seizures, we also consider your adherence to prescribed treatment (see 111.00C). When we determine the number of seizures you have had in the specified period, we will:
a. Count multiple seizures occurring in a 24-hour period as one seizure.
b. Count status epilepticus (a continuous series of seizures without return to consciousness between seizures) as one seizure.
c. Count a dyscognitive seizure that progresses into a generalized tonic-clonic seizure as one generalized tonic-clonic seizure.
d. We do not count seizures that occur during a period when you are not adhering to prescribed treatment without good reason. When we determine that you had a good reason for not adhering to prescribed treatment, we will consider your physical, mental, educational, and communicative limitations (including any language barriers). We will consider you to have good reason for not following prescribed treatment if, for example, the treatment is very risky for you due to its consequences or unusual nature, or if you are unable to afford prescribed treatment that you are willing to accept, but for which no free community resources are available. We will follow guidelines found in our policy, such as § 416.930(c) of this chapter, when we determine whether you have a good reason for not adhering to prescribed treatment.
e. We do not count psychogenic nonepileptic seizures or pseudoseizures under 111.02.We evaluate these seizures under the mental disorders body system, 112.00.
5. Electroencephalography (EEG) testing. We do not require EEG test results; therefore, we will not purchase them. However, if EEG test results are available in your medical records, we will evaluate them in the context of the other evidence in your case record.
G. What is vascular insult to the brain, and how do we evaluate it under 111.04?
1. Vascular insult to the brain (cerebrum, cerebellum, or brainstem), commonly referred to as stroke or cerebrovascular accident (CVA), is brain cell death caused by an interruption of blood flow within or leading to the brain, or by a hemorrhage from a ruptured blood vessel or aneurysm in the brain. If you have a vision impairment resulting from your vascular insult, we may evaluate that impairment under the special senses body system, 102.00.
2. We generally need evidence from at least 3 months after the vascular insult to determine whether you have disorganization of motor function under 111.04. In some cases, evidence of your vascular insult is sufficient to allow your claim within 3 months post-vascular insult. If we are unable to allow your claim within 3 months after your vascular insult, we will defer adjudication of the claim until we obtain evidence of your neurological disorder at least 3 months post-vascular insult.
H. What are benign brain tumors, and how do we evaluate them under 111.05? Benign brain tumors are noncancerous (nonmalignant) abnormal growths of tissue in or on the brain that invade healthy brain tissue or apply pressure on the brain or cranial nerves. We evaluate their effects on your functioning as discussed in 111.00D. We evaluate malignant brain tumors under the cancer body system in 113.00. If you have a vision impairment resulting from your benign brain tumor, we may evaluate that impairment under the special senses body system, 102.00.
I. What is cerebral palsy, and how do we evaluate it under 111.07?
1. Cerebral palsy (CP) is a term that describes a group of static, nonprogressive disorders caused by abnormalities within the brain that disrupt the brain's ability to control movement, muscle coordination, and posture. The resulting motor deficits manifest very early in a child's development, with delayed or abnormal progress in attaining developmental milestones; deficits may become more obvious as the child grows and matures over time.
2. We evaluate your signs and symptoms, such as ataxia, spasticity, flaccidity, athetosis, chorea, and difficulty with precise movements when we determine your ability to stand up, balance, walk, or perform fine and gross motor movements. We will also evaluate your signs, such as dysarthria and apraxia of speech, and receptive and expressive language problems when we determine your ability to communicate.
3. We will consider your other impairments or signs and symptoms that develop secondary to the disorder, such as post-impairment syndrome (a combination of pain, fatigue, and weakness due to muscle abnormalities); overuse syndromes (repetitive motion injuries); arthritis; abnormalities of proprioception (perception of the movements and position of the body); abnormalities of stereognosis (perception and identification of objects by touch); learning problems; anxiety; and depression.
J. What are spinal cord disorders, and how do we evaluate them under 111.08?
1. Spinal cord disorders may be congenital or caused by injury to the spinal cord. Motor signs and symptoms of spinal cord disorders include paralysis, flaccidity, spasticity, and weakness.
2. Spinal cord disorders with complete loss of function (111.08A) addresses spinal cord disorders that result in complete lack of motor, sensory, and autonomic function of the affected part(s) of the body.
3. Spinal cord disorders with disorganization of motor function (111.08B) addresses spinal cord disorders that result in less than complete loss of function of the affected part(s) of the body, reducing, but not eliminating, motor, sensory, and autonomic function.
4. When we evaluate your spinal cord disorder, we generally need evidence from at least 3 months after your symptoms began in order to evaluate your disorganization of motor function. In some cases, evidence of your spinal cord disorder may be sufficient to allow your claim within 3 months after the spinal cord disorder. If the medical evidence demonstrates total cord transection causing a loss of motor and sensory functions below the level of injury, we will not wait 3 months but will make the allowance decision immediately.
K. What are communication impairments associated with neurological disorders, and how do we evaluate them under 111.09?
1. Communication impairments result from medically determinable neurological disorders that cause dysfunction in the parts of the brain responsible for speech and language. Under 111.09, we must have recent comprehensive evaluation including all areas of affective and effective communication, performed by a qualified professional, to document a communication impairment associated with a neurological disorder.
2. Under 111.09A, we need documentation from a qualified professional that your neurological disorder has resulted in a speech deficit that significantly affects your ability to communicate. Significantly affects means that you demonstrate a serious limitation in communicating, and a person who is unfamiliar with you cannot easily understand or interpret your speech.
3. Under 111.09B, we need documentation from a qualified professional that shows that your neurological disorder has resulted in a comprehension deficit that results in ineffective verbal communication for your age. For the purposes of 111.09B, comprehension deficit means a deficit in receptive language. Ineffective verbal communication means that you demonstrate serious limitation in your ability to communicate orally on the same level as other children of the same age and level of development.
4. Under 111.09C, we need documentation of a neurological disorder that has resulted in hearing loss. Your hearing loss will be evaluated under listing 102.10 or 102.11.
5. We evaluate speech deficits due to non-neurological disorders under 2.09.
L. What are neurodegenerative disorders of the central nervous system, such as Juvenile-onset Huntington's disease and Friedreich's ataxia, and how do we evaluate them under 111.17? Neurodegenerative disorders of the central nervous system are disorders characterized by progressive and irreversible degeneration of neurons or their supporting cells. Over time, these disorders impair many of the body's motor or cognitive and other mental functions. We consider neurodegenerative disorders of the central nervous system under 111.17 that we do not evaluate elsewhere in section 111.00, such as juvenile-onset Huntington's disease (HD) and Friedreich's ataxia. When these disorders result in solely cognitive and other mental functional limitations, we will evaluate the disorder under the mental disorder listings, 112.00.
M. What is traumatic brain injury, and how do we evaluate it under 111.18?
1. Traumatic brain injury (TBI) is damage to the brain resulting from skull fracture, collision with an external force leading to a closed head injury, or penetration by an object that enters the skull and makes contact with brain tissue. We evaluate a TBI that results in coma or persistent vegetative state (PVS) under 111.20.
2. We generally need evidence from at least 3 months after the TBI to evaluate whether you have disorganization of motor function under 111.18. In some cases, evidence of your TBI is sufficient to determine disability. If we are unable to allow your claim within 3 months post-TBI, we will defer adjudication of the claim until we obtain evidence of your neurological disorder at least 3 months post-TBI. If a finding of disability still is not possible at that time, we will again defer adjudication of the claim until we obtain evidence at least 6 months after your TBI.
N. What are coma and persistent vegetative state, and how do we evaluate them under 111.20? Coma is a state of unconsciousness in which a child does not exhibit a sleep/wake cycle, and is unable to perceive or respond to external stimuli. Children who do not fully emerge from coma may progress into persistent vegetative state (PVS). PVS is a condition of partial arousal in which a child may have a low level of consciousness but is still unable to react to external stimuli. In contrast to coma, a child in a PVS retains sleep/wake cycles and may exhibit some key lower brain functions, such as spontaneous movement, opening and moving eyes, and grimacing. Coma or PVS may result from a TBI, a nontraumatic insult to the brain (such as a vascular insult, infection, or brain tumor), or a neurodegenerative or metabolic disorder. Medically induced comas should be considered under the section pertaining to the underlying reason the coma was medically induced and not under this section.
O. What is multiple sclerosis, and how do we evaluate it under 111.21?
1. Multiple sclerosis (MS) is a chronic, inflammatory, degenerative disorder that damages the myelin sheath surrounding the nerve fibers in the brain and spinal cord. The damage disrupts the normal transmission of nerve impulses within the brain and between the brain and other parts of the body causing impairment in muscle coordination, strength, balance, sensation, and vision. There are several forms of MS, ranging from slightly to highly aggressive. Milder forms generally involve acute attacks (exacerbations) with partial or complete recovery from signs and symptoms (remissions). Aggressive forms generally exhibit a steady progression of signs and symptoms with few or no remissions. The effects of all forms vary from child to child.
2. We evaluate your signs and symptoms, such as flaccidity, spasticity, spasms, incoordination, imbalance, tremor, physical fatigue, muscle weakness, dizziness, tingling, and numbness when we determine your ability to stand up, balance, walk, or perform fine and gross motor movements, such as using your arms, hands, and fingers. If you have a vision impairment resulting from your MS, we may evaluate that impairment under the special senses body system, 102.00.
P. What are motor neuron disorders, and how do we evaluate them under 111.22? Motor neuron disorders are progressive neurological disorders that destroy the cells that control voluntary muscle activity, such as walking, breathing, swallowing, and speaking. The most common motor neuron disorders in children are progressive bulbar palsy and spinal muscular dystrophy syndromes. We evaluate the effects of these disorders on motor functioning or bulbar and neuromuscular functioning.
Q. How do we consider symptoms of fatigue in these listings? Fatigue is one of the most common and limiting symptoms of some neurological disorders, such as multiple sclerosis and myasthenia gravis. These disorders may result in physical fatigue (lack of muscle strength) or mental fatigue (decreased awareness or attention). When we evaluate your fatigue, we will consider the intensity, persistence, and effects of fatigue on your functioning. This may include information such as the clinical and laboratory data and other objective evidence concerning your neurological deficit, a description of fatigue considered characteristic of your disorder, and information about your functioning. We consider the effects of physical fatigue on your ability to stand up, balance, walk, or perform fine and gross motor movements using the criteria described in 111.00D.
R. How do we evaluate your neurological disorder when it does not meet one of these listings?
1. If your neurological disorder does not meet the criteria of any of these listings, we must also consider whether your impairment(s) meets the criteria of a listing in another body system. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. See § 416.926 of this chapter.
2. If your impairment(s) does not meet or medically equal a listing, we will consider whether your impairment(s) functionally equals the listings. See § 416.926a of this chapter.
3. We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
111.01 Category of Impairments, Neurological Disorders111.02 Epilepsy, documented by a detailed description of a typical seizure and characterized by A or B:
A. Generalized tonic-clonic seizures (see 111.00F1a), occurring at least once a month for at least 3 consecutive months (see 111.00F4) despite adherence to prescribed treatment (see 111.00C); or
B. Dyscognitive seizures (see 111.00F1b) or absence seizures (see 111.00F1c), occurring at least once a week for at least 3 consecutive months (see 111.00F4) despite adherence to prescribed treatment (see 111.00C).
111.03 [Reserved]
111.04 Vascular insult to the brain, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities, persisting for at least 3 consecutive months after the insult.
111.05 Benign brain tumors, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.06 [Reserved]
111.07 Cerebral palsy, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.08 Spinal cord disorders, characterized by A or B:
A. Complete loss of function, as described in 111.00J2, persisting for 3 consecutive months after the disorder (see 111.00J4); or
B. Disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities persisting for 3 consecutive months after the disorder (see 111.00J4).
111.09 Communication impairment, associated with documented neurological disorder and one of the following:
A. Documented speech deficit that significantly affects (see 111.00K1) the clarity and content of the speech; or
B. Documented comprehension deficit resulting in ineffective verbal communication (see 111.00K2) for age; or
C. Impairment of hearing as described under the criteria in 102.10 or 102.11.
111.10 [Reserved]
111.11 [Reserved]
111.12 Myasthenia gravis, characterized by A or B despite adherence to prescribed treatment for at least 3 months (see 111.00C):
A. Disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Bulbar and neuromuscular dysfunction (see 111.00E), resulting in:
1. One myasthenic crisis requiring mechanical ventilation; or
2. Need for supplemental enteral nutrition via a gastrostomy or parenteral nutrition via a central venous catheter.
111.13 Muscular dystrophy, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.14 Peripheral neuropathy, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.15 [Reserved]
111.16 [Reserved]
111.17 Neurodegenerative disorders of the central nervous system, such as Juvenile-onset Huntington's disease and Friedreich's ataxia, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.18 Traumatic brain injury, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities, persisting for at least 3 consecutive months after the injury.
111.19 [Reserved]
111.20 Coma or persistent vegetative state, persisting for at least 1 month.
111.21 Multiple sclerosis, characterized by disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities.
111.22 Motor neuron disorders, characterized by A or B:
A. Disorganization of motor function in two extremities (see 111.00D1), resulting in an extreme limitation (see 111.00D2) in the ability to stand up from a seated position, balance while standing or walking, or use the upper extremities; or
B. Bulbar and neuromuscular dysfunction (see 111.00E), resulting in:
1. Acute respiratory failure requiring invasive mechanical ventilation; or
2. Need for supplemental enteral nutrition via a gastrostomy or parenteral nutrition via a central venous catheter.
112.00 Mental DisordersA. How are the listings for mental disorders for children arranged, and what do they require?
1. The listings for mental disorders for children are arranged in 12 categories: neurocognitive disorders (112.02); schizophrenia spectrum and other psychotic disorders (112.03); depressive, bipolar and related disorders (112.04); intellectual disorder (112.05); anxiety and obsessive-compulsive disorders (112.06); somatic symptom and related disorders (112.07); personality and impulse-control disorders (112.08); autism spectrum disorder (112.10); neurodevelopmental disorders (112.11); eating disorders (112.13); developmental disorders in infants and toddlers (112.14); and trauma- and stressor-related disorders (112.15). All of these listings, with the exception of 112.14, apply to children from age three to attainment of age 18. Listing 112.14 is for children from birth to attainment of age 3.
2. Listings 112.07, 112.08, 112.10, 112.11, 112.13, and 112.14 have two paragraphs, designated A and B; your mental disorder must satisfy the requirements of both paragraphs A and B. Listings 112.02, 112.03, 112.04, 112.06, and 112.15 have three paragraphs, designated A, B, and C; your mental disorder must satisfy the requirements of both paragraphs A and B, or the requirements of both paragraphs A and C. Listing 112.05 has two paragraphs that are unique to that listing (see 112.00A3); your mental disorder must satisfy the requirements of either paragraph A or paragraph B.
a. Paragraph A of each listing (except 112.05) includes the medical criteria that must be present in your medical evidence.
b. Paragraph B of each listing (except 112.05) provides the functional criteria we assess to evaluate how your mental disorder limits your functioning. For children ages 3 to 18, these criteria represent the areas of mental functioning a child uses to perform age-appropriate activities. They are: understand, remember, or apply information; interact with others; concentrate, persist, or maintain pace; and adapt or manage oneself. (See 112.00I for a discussion of the criteria for children from birth to attainment of age 3 under 112.14.) We will determine the degree to which your medically determinable mental impairment affects the four areas of mental functioning and your ability to function age-appropriately in a manner comparable to that of other children your age who do not have impairments. (Hereinafter, the words “age-appropriately” incorporate the qualifying statement, “in a manner comparable to that of other children your age who do not have impairments.”) To satisfy the paragraph B criteria, your mental disorder must result in “extreme” limitation of one, or “marked” limitation of two, of the four areas of mental functioning. (When we refer to “paragraph B criteria” or “area[s] of mental functioning” in the introductory text of this body system, we mean the criteria in paragraph B of every listing except 112.05 and 112.14.)
c. Paragraph C of listings 112.02, 112.03, 112.04, 112.06, and 112.15 provides the criteria we use to evaluate “serious and persistent mental disorders.” To satisfy the paragraph C criteria, your mental disorder must be “serious and persistent”; that is, there must be a medically documented history of the existence of the disorder over a period of at least 2 years, and evidence that satisfies the criteria in both C1 and C2 (see 112.00G). (When we refer to “paragraph C” or “the paragraph C criteria” in the introductory text of this body system, we mean the criteria in paragraph C of listings 112.02, 112.03, 112.04, 112.06, and 112.15.)
3. Listing 112.05 has two paragraphs, designated A and B, that apply to only intellectual disorder. Each paragraph requires that you have significantly subaverage general intellectual functioning and significant deficits in current adaptive functioning.
B. Which mental disorders do we evaluate under each listing category for children?
1. Neurocognitive disorders (112.02).
a. These disorders are characterized in children by a clinically significant deviation in normal cognitive development or by a decline in cognitive functioning. Symptoms and signs may include, but are not limited to, disturbances in memory, executive functioning (that is, higher-level cognitive processes; for example, regulating attention, planning, inhibiting responses, decision-making), visual-spatial functioning, language and speech, perception, insight, and judgment.
b. Examples of disorders that we evaluate in this category include major neurocognitive disorder; mental impairments resulting from medical conditions such as a metabolic disease (for example, juvenile Tay-Sachs disease), human immunodeficiency virus infection, vascular malformation, progressive brain tumor, or traumatic brain injury; or substance-induced cognitive disorder associated with drugs of abuse, medications, or toxins. (We evaluate neurological disorders under that body system (see 111.00). We evaluate cognitive impairments that result from neurological disorders under 112.02 if they do not satisfy the requirements in 111.00. We evaluate catastrophic genetic disorders under listings in 110.00, 111.00, or 112.00, as appropriate. We evaluate genetic disorders that are not catastrophic under the affected body system(s).)
c. This category does not include the mental disorders that we evaluate under intellectual disorder (112.05), autism spectrum disorder (112.10), and neurodevelopmental disorders (112.11).
2. Schizophrenia spectrum and other psychotic disorders (112.03).
a. These disorders are characterized by delusions, hallucinations, disorganized speech, or grossly disorganized or catatonic behavior, causing a clinically significant decline in functioning. Symptoms and signs may include, but are not limited to, inability to initiate and persist in goal-directed activities, social withdrawal, flat or inappropriate affect, poverty of thought and speech, loss of interest or pleasure, disturbances of mood, odd beliefs and mannerisms, and paranoia.
b. Examples of disorders that we evaluate in this category include schizophrenia, schizoaffective disorder, delusional disorder, and psychotic disorder due to another medical condition.
3. Depressive, bipolar and related disorders (112.04).
a. These disorders are characterized by an irritable, depressed, elevated, or expansive mood, or by a loss of interest or pleasure in all or almost all activities, causing a clinically significant decline in functioning. Symptoms and signs may include, but are not limited to, feelings of hopelessness or guilt, suicidal ideation, a clinically significant change in body weight or appetite, sleep disturbances, an increase or decrease in energy, psychomotor abnormalities, disturbed concentration, pressured speech, grandiosity, reduced impulse control, sadness, euphoria, and social withdrawal. Depending on a child's age and developmental stage, certain features, such as somatic complaints, irritability, anger, aggression, and social withdrawal may be more commonly present than other features.
b. Examples of disorders that we evaluate in this category include bipolar disorders (I or II), cyclothymic disorder, disruptive mood dysregulation disorder, major depressive disorder, persistent depressive disorder (dysthymia), and bipolar or depressive disorder due to another medical condition.
4. Intellectual disorder (112.05).
a. This disorder is characterized by significantly subaverage general intellectual functioning and significant deficits in current adaptive functioning. Signs may include, but are not limited to, poor conceptual, social, or practical skills evident in your adaptive functioning.
b. The disorder that we evaluate in this category may be described in the evidence as intellectual disability, intellectual developmental disorder, or historically used terms such as “mental retardation.”
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (112.02), autism spectrum disorder (112.10), or neurodevelopmental disorders (112.11).
5. Anxiety and obsessive-compulsive disorders (112.06).
a. These disorders are characterized by excessive anxiety, worry, apprehension, and fear, or by avoidance of feelings, thoughts, activities, objects, places, or people. Symptoms and signs may include, but are not limited to, restlessness, difficulty concentrating, hyper-vigilance, muscle tension, sleep disturbance, fatigue, panic attacks, obsessions and compulsions, constant thoughts and fears about safety, and frequent physical complaints. Depending on a child's age and developmental stage, other features may also include refusal to go to school, academic failure, frequent stomachaches and other physical complaints, extreme worries about sleeping away from home, being overly clinging, and exhibiting tantrums at times of separation from caregivers.
b. Examples of disorders that we evaluate in this category include separation anxiety disorder, social anxiety disorder, panic disorder, generalized anxiety disorder, agoraphobia, and obsessive-compulsive disorder.
c. This category does not include the mental disorders that we evaluate under trauma- and stressor-related disorders (112.15).
6. Somatic symptom and related disorders (112.07).
a. These disorders are characterized by physical symptoms or deficits that are not intentionally produced or feigned, and that, following clinical investigation, cannot be fully explained by a general medical condition, another mental disorder, the direct effects of a substance, or a culturally sanctioned behavior or experience. Symptoms and signs may include, but are not limited to, pain and other abnormalities of sensation, gastrointestinal symptoms, fatigue, abnormal motor movement, pseudoseizures, and pseudoneurological symptoms, such as blindness or deafness.
b. Examples of disorders that we evaluate in this category include somatic symptom disorder and conversion disorder.
7. Personality and impulse-control disorders (112.08).
a. These disorders are characterized by enduring, inflexible, maladaptive, and pervasive patterns of behavior. Onset may occur in childhood but more typically occurs in adolescence or young adulthood. Symptoms and signs may include, but are not limited to, patterns of distrust, suspiciousness, and odd beliefs; social detachment, discomfort, or avoidance; hypersensitivity to negative evaluation; an excessive need to be taken care of; difficulty making independent decisions; a preoccupation with orderliness, perfectionism, and control; and inappropriate, intense, impulsive anger and behavioral expression grossly out of proportion to any external provocation or psychosocial stressors.
b. Examples of disorders that we evaluate in this category include paranoid, schizoid, schizotypal, borderline, avoidant, dependent, obsessive-compulsive personality disorders, and intermittent explosive disorder.
8. Autism spectrum disorder (112.10).
a. These disorders are characterized by qualitative deficits in the development of reciprocal social interaction, verbal and nonverbal communication skills, and symbolic or imaginative play; restricted repetitive and stereotyped patterns of behavior, interests, and activities; and stagnation of development or loss of acquired skills. Symptoms and signs may include, but are not limited to, abnormalities and unevenness in the development of cognitive skills; unusual responses to sensory stimuli; and behavioral difficulties, including hyperactivity, short attention span, impulsivity, aggressiveness, or self-injurious actions.
b. Examples of disorders that we evaluate in this category include autism spectrum disorder with or without accompanying intellectual impairment, and autism spectrum disorder with or without accompanying language impairment.
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (112.02), intellectual disorder (112.05), and neurodevelopmental disorders (112.11).
9. Neurodevelopmental disorders (112.11).
a. These disorders are characterized by onset during the developmental period, that is, during childhood or adolescence, although sometimes they are not diagnosed until adulthood. Symptoms and signs may include, but are not limited to, underlying abnormalities in cognitive processing (for example, deficits in learning and applying verbal or nonverbal information, visual perception, memory, or a combination of these); deficits in attention or impulse control; low frustration tolerance; excessive or poorly planned motor activity; difficulty with organizing (time, space, materials, or tasks); repeated accidental injury; and deficits in social skills. Symptoms and signs specific to tic disorders include sudden, rapid, recurrent, non-rhythmic, motor movement or vocalization.
b. Examples of disorders that we evaluate in this category include specific learning disorder, borderline intellectual functioning, and tic disorders (such as Tourette syndrome).
c. This category does not include the mental disorders that we evaluate under neurocognitive disorders (112.02), autism spectrum disorder (112.10), or personality and impulse-control disorders (112.08).
10. Eating disorders (112.13).
a. These disorders are characterized in young children by persistent eating of nonnutritive substances or repeated episodes of regurgitation and re-chewing of food, or by persistent failure to consume adequate nutrition by mouth. In adolescence, these disorders are characterized by disturbances in eating behavior and preoccupation with, and excessive self-evaluation of, body weight and shape. Symptoms and signs may include, but are not limited to, failure to make expected weight gains; restriction of energy consumption when compared with individual requirements; recurrent episodes of binge eating or behavior intended to prevent weight gain, such as self-induced vomiting, excessive exercise, or misuse of laxatives; mood disturbances, social withdrawal, or irritability; amenorrhea; dental problems; abnormal laboratory findings; and cardiac abnormalities.
b. Examples of disorders that we evaluate in this category include anorexia nervosa, bulimia nervosa, binge-eating disorder, and avoidant/restrictive food disorder.
11. Developmental disorders in infants and toddlers (112.14).
a. Developmental disorders are characterized by a delay or deficit in the development of age-appropriate skills, or a loss of previously acquired skills, involving motor planning and control, learning, relating and communicating, and self-regulating.
b. Examples of disorders that we evaluate in this category include developmental coordination disorder, separation anxiety disorder, autism spectrum disorder, and regulation disorders of sensory processing (difficulties in regulating emotions, behaviors, and motor abilities in response to sensory stimulation). Some infants and toddlers may have only a general diagnosis of “developmental delay.”
c. This category does not include eating disorders related to low birth weight and failure to thrive, which we evaluate under that body system (100.00).
12. Trauma- and stressor-related disorders (112.15).
a. These disorders are characterized by experiencing or witnessing a traumatic or stressful event, or learning of a traumatic event occurring to a close family member or close friend, and the psychological aftermath of clinically significant effects on functioning. Symptoms and signs may include, but are not limited to, distressing memories, dreams, and flashbacks related to the trauma or stressor; avoidant or withdrawn behavior; constriction of play and significant activities; increased frequency of negative emotional states (for example, fear, sadness) or reduced expression of positive emotions (for example, satisfaction, affection); anxiety; irritability; aggression; exaggerated startle response; difficulty concentrating; sleep disturbance; and a loss of previously acquired developmental skills.
b. Examples of disorders that we evaluate in this category include posttraumatic stress disorder, reactive attachment disorder, and other specified trauma- and stressor-related disorders (such as adjustment-like disorders with prolonged duration without prolonged duration of stressor).
c. This category does not include the mental disorders that we evaluate under anxiety and obsessive-compulsive disorders (112.06), and cognitive impairments that result from neurological disorders, such as a traumatic brain injury, which we evaluate under neurocognitive disorders (112.02).
C. What evidence do we need to evaluate your mental disorder?
1. General. We need objective medical evidence from an acceptable medical source to establish that you have a medically determinable mental disorder. We also need evidence to assess the severity of your mental disorder and its effects on your ability to function age-appropriately. We will determine the extent and kinds of evidence we need from medical and nonmedical sources based on the individual facts about your disorder. For additional evidence requirements for intellectual disorder (112.05), see 112.00H. For our basic rules on evidence, see §§ 416.912, 416.913, and 416.920b of this chapter. For our rules on evaluating medical opinions, see §§ 416.1520c and 416.927 of this chapter. For our rules on evidence about your symptoms, see § 416.929 of this chapter.
2. Evidence from medical sources. We will consider all relevant medical evidence about your disorder from your physician, psychologist, and other medical sources, which include health care providers such as physician assistants, psychiatric nurse practitioners, licensed clinical social workers, and clinical mental health counselors. Evidence from your medical sources may include:
a. Your reported symptoms.
b. Your developmental, medical, psychiatric, and psychological history.
c. The results of physical or mental status examinations, structured clinical interviews, psychiatric or psychological rating scales, measures of adaptive functioning, or other clinical findings.
d. Developmental assessments, psychological testing, imaging results, or other laboratory findings.
e. Your diagnosis.
f. The type, dosage, and beneficial effects of medications you take.
g. The type, frequency, duration, and beneficial effects of therapy you receive.
h. Side effects of medication or other treatment that limit your ability to function.
i. Your clinical course, including changes in your medication, therapy, or other treatment, and the time required for therapeutic effectiveness.
j. Observations and descriptions of how you function during examinations or therapy.
k. Information about sensory, motor, or speech abnormalities, or about your cultural background (for example, language or customs) that may affect an evaluation of your mental disorder.
l. The expected duration of your symptoms and signs and their effects on your ability to function age-appropriately, both currently and in the future.
3. Evidence from you and people who know you. We will consider all relevant evidence about your mental disorder and your daily functioning that we receive from you and from people who know you. If you are too young or unable to describe your symptoms and your functioning, we will ask for a description from the person who is most familiar with you. We will ask about your symptoms, your daily functioning, and your medical treatment. We will ask for information from third parties who can tell us about your mental disorder, but we must have permission to do so. This evidence may include information from your family, caregivers, teachers, other educators, neighbors, clergy, case managers, social workers, shelter staff, or other community support and outreach workers. We will consider whether your statements and the statements from third parties are consistent with the medical and other evidence we have.
4. Evidence from early intervention programs, school, vocational training, work, and work-related programs.
a. Early intervention programs. You may receive services in an Early Intervention Program (EIP) to help you with your developmental needs. If so, we will consider information from your Individualized Family Service Plan (IFSP) and the early intervention specialists who help you.
b. School. You may receive special education or related services at your preschool or school. If so, we will try to obtain information from your school sources when we need it to assess how your mental disorder affects your ability to function. Examples of this information include your Individualized Education Programs (IEPs), your Section 504 plans, comprehensive evaluation reports, school-related therapy progress notes, information from your teachers about how you function in a classroom setting, and information from special educators, nurses, school psychologists, and occupational, physical, and speech/language therapists about any special education services or accommodations you receive at school.
c. Vocational training, work, and work-related programs. You may have recently participated in or may still be participating in vocational training, work-related programs, or work activity. If so, we will try to obtain information from your training program or your employer when we need it to assess how your mental disorder affects your ability to function. Examples of this information include training or work evaluations, modifications to your work duties or work schedule, and any special supports or accommodations you have required or now require in order to work. If you have worked or are working through a community mental health program, sheltered or supported work program, rehabilitation program, or transitional employment program, we will consider the type and degree of support you have received or are receiving in order to work (see 112.00D).
5. Need for longitudinal evidence.
a. General. Longitudinal medical evidence can help us learn how you function over time, and help us evaluate any variations in the level of your functioning. We will request longitudinal evidence of your mental disorder when your medical providers have records concerning you and your mental disorder over a period of months or perhaps years (see § 416.912(d) of this chapter).
b. Non-medical sources of longitudinal evidence. Certain situations, such as chronic homelessness, may make it difficult for you to provide longitudinal medical evidence. If you have a severe mental disorder, you will probably have evidence of its effects on your functioning over time, even if you have not had an ongoing relationship with the medical community or are not currently receiving treatment. For example, family members, caregivers, teachers, neighbors, former employers, social workers, case managers, community support staff, outreach workers, or government agencies may be familiar with your mental health history. We will ask for information from third parties who can tell us about your mental disorder, but you must give us permission to do so.
c. Absence of longitudinal evidence. In the absence of longitudinal evidence, we will use current objective medical evidence and all other relevant evidence available to us in your case record to evaluate your mental disorder. If we purchase a consultative examination to document your disorder, the record will include the results of that examination (see § 416.914 of this chapter). We will take into consideration your medical history, symptoms, clinical and laboratory findings, and medical source opinions. If you do not have longitudinal evidence, the current evidence alone may not be sufficient or appropriate to show that you have a disorder that meets the criteria of one of the mental disorders listings. In that case, we will follow the rules in 112.00K.
6. Evidence of functioning in unfamiliar situations or supportive situations.
a. Unfamiliar situations. We recognize that evidence about your functioning in unfamiliar situations does not necessarily show how you would function on a sustained basis in a school or other age-appropriate setting. In one-time, time-limited, or other unfamiliar situations, you may function differently than you do in familiar situations. In unfamiliar situations, you may appear more, or less, limited than you do on a daily basis and over time.
b. Supportive situations. Your ability to function in settings that are highly structured, or that are less demanding or more supportive than settings in which children your age without impairments typically function, does not necessarily demonstrate your ability to function age-appropriately.
c. Our assessment. We must assess your ability to function age-appropriately by evaluating all the evidence, such as reports about your functioning from third parties who are familiar with you, with an emphasis on how well you can initiate, sustain, and complete age-appropriate activities despite your impairment(s), compared to other children your age who do not have impairments.
D. How do we consider psychosocial supports, structured settings, living arrangements, and treatment when we evaluate the functioning of children?
1. General. Psychosocial supports, structured settings, and living arrangements, including assistance from your family or others, may help you by reducing the demands made on you. In addition, treatment you receive may reduce your symptoms and signs and possibly improve your functioning, or may have side effects that limit your functioning. Therefore, when we evaluate the effects of your mental disorder and rate the limitation of your areas of mental functioning, we will consider the kind and extent of supports you receive, the characteristics of any structured setting in which you spend your time (compared to children your age without impairments), and the effects of any treatment. This evidence may come from reports about your functioning from third parties who are familiar with you, and other third-party statements or information. Following are some examples of the supports you may receive:
a. You receive help from family members or other people in ways that children your age without impairments typically do not need in order to function age-appropriately. For example, an aide may accompany you on the school bus to help you control your actions or to monitor you to ensure you do not injure yourself or others.
b. You receive one-on-one assistance in your classes every day; or you have a full-time personal aide who helps you to function in your classroom; or you are a student in a self-contained classroom; or you attend a separate or alternative school where you receive special education services.
c. You participate in a special education or vocational training program, or a psychosocial rehabilitation day treatment or community support program, where you receive training in daily living and entry-level work skills.
d. You participate in a sheltered, supported, or transitional work program, or in a competitive employment setting with the help of a job coach or supervisor.
e. You receive comprehensive “24/7 wrap-around” mental health services while living in a group home or transitional housing, while participating in a semi-independent living program, or while living at home.
f. You live in a residential school, hospital, or other institution with 24-hour care.
g. You receive assistance from a crisis response team, social workers, or community mental health workers who help you meet your physical needs, and who may also represent you in dealings with government or community social services.
2. How we consider different levels of support and structure in psychosocial rehabilitation programs.
a. Psychosocial rehabilitation programs are based on your specific needs. Therefore, we cannot make any assumptions about your mental disorder based solely on the fact that you are associated with such a program. We must know the details of the program(s) in which you are involved and the pattern(s) of your involvement over time.
b. The kinds and levels of supports and structures in psychosocial rehabilitation programs typically occur on a scale of “most restrictive” to “least restrictive.” Participation in a psychosocial rehabilitation program at the most restrictive level would suggest greater limitation of your areas of mental functioning than would participation at a less restrictive level. The length of time you spend at different levels in a program also provides information about your functioning. For example, you could begin participation at the most restrictive crisis intervention level but gradually improve to the point of readiness for a lesser level of support and structure and, if you are an older adolescent, possibly some form of employment.
3. How we consider the help or support you receive.
a. We will consider the complete picture of your daily functioning, including the kinds, extent, and frequency of help and support you receive, when we evaluate your mental disorder and determine whether you are able to use the four areas of mental functioning age-appropriately. The fact that you have done, or currently do, some routine activities without help or support does not necessarily mean that you do not have a mental disorder or that you are not disabled. For example, you may be able to take age-appropriate care of your personal needs, or you may be old enough and able to cook, shop, and take public transportation. You may demonstrate both strengths and deficits in your daily functioning.
b. You may receive various kinds of help and support from others that enable you to do many things that, because of your mental disorder, you might not be able to do independently. Your daily functioning may depend on the special contexts in which you function. For example, you may spend your time among only familiar people or surroundings, in a simple and steady routine or an unchanging environment, or in a highly structured classroom or alternative school. However, this does not necessarily show whether you would function age-appropriately without those supports or contexts. (See 112.00H for further discussion of these issues regarding significant deficits in adaptive functioning for the purpose of 112.05.)
4. How we consider treatment. We will consider the effect of any treatment on your functioning when we evaluate your mental disorder. Treatment may include medication(s), psychotherapy, or other forms of intervention, which you receive in a doctor's office, during a hospitalization, or in a day program at a hospital or outpatient treatment program. With treatment, you may not only have your symptoms and signs reduced, but may also be able to function age-appropriately. However, treatment may not resolve all of the limitations that result from your mental disorder, and the medications you take or other treatment you receive for your disorder may cause side effects that limit your mental or physical functioning. For example, you may experience drowsiness, blunted affect, memory loss, or abnormal involuntary movements.
E. What are the paragraph B criteria for children age 3 to the attainment of age 18?
1. Understand, remember, or apply information (paragraph B1). This area of mental functioning refers to the abilities to learn, recall, and use information to perform age-appropriate activities. Examples include: Understanding and learning terms, instructions, procedures; following one- or two-step oral instructions to carry out a task; describing an activity to someone else; asking and answering questions and providing explanations; recognizing a mistake and correcting it; identifying and solving problems; sequencing multi-step activities; and using reason and judgment to make decisions. These examples illustrate the nature of the area of mental functioning. We do not require documentation of all of the examples. How you manifest this area of mental functioning and your limitations in using it depends, in part, on your age.
2. Interact with others (paragraph B2). This area of mental functioning refers to the abilities to relate to others age-appropriately at home, at school, and in the community. Examples include: Engaging in interactive play; cooperating with others; asking for help when needed; initiating and maintaining friendships; handling conflicts with others; stating own point of view; initiating or sustaining conversation; understanding and responding to social cues (physical, verbal, emotional); responding to requests, suggestions, criticism, correction, and challenges; and keeping social interactions free of excessive irritability, sensitivity, argumentativeness, or suspiciousness. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples. How you manifest this area of mental functioning and your limitations in using it depends, in part, on your age.
3. Concentrate, persist, or maintain pace (paragraph B3). This area of mental functioning refers to the abilities to focus attention on activities and stay on task age-appropriately. Examples include: Initiating and performing an activity that you understand and know how to do; engaging in an activity at home or in school at an appropriate and consistent pace; completing tasks in a timely manner; ignoring or avoiding distractions while engaged in an activity or task; changing activities without being disruptive; engaging in an activity or task close to or with others without interrupting or distracting them; sustaining an ordinary routine and regular attendance at school; and engaging in activities at home, school, or in the community without needing an unusual amount of rest. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples. How you manifest this area of mental functioning and your limitations in using it depends, in part, on your age.
4. Adapt or manage oneself (paragraph B4). This area of mental functioning refers to the abilities to regulate emotions, control behavior, and maintain well-being in age-appropriate activities and settings. Examples include: Responding to demands; adapting to changes; managing your psychologically based symptoms; distinguishing between acceptable and unacceptable performance in community- or school-related activities; setting goals; making plans independently of others; maintaining personal hygiene; and protecting yourself from harm and exploitation by others. These examples illustrate the nature of this area of mental functioning. We do not require documentation of all of the examples. How you manifest this area of mental functioning and your limitations in using it depends, in part, on your age.
F. How do we use the paragraph B criteria to evaluate mental disorders in children?
1. General. We use the paragraph B criteria to rate the degree of your limitations. We consider only the limitations that result from your mental disorder(s). We will determine whether you are able to use each of the paragraph B areas of mental functioning in age-appropriate activities in a manner comparable to that of other children your age who do not have impairments. We will consider, for example, the range of your activities and whether they are age-appropriate; how well you can initiate, sustain, and complete your activities; the kinds and frequency of help or supervision you receive; and the kinds of structured or supportive settings you need in order to function age-appropriately (see 112.00D).
2. Degrees of limitation. We evaluate the effects of your mental disorder on each of the four areas of mental functioning. To satisfy the paragraph B criteria, your mental disorder must result in extreme limitation of one, or marked limitation of two, paragraph B areas of mental functioning. See §§ 416.925(b)(2)(ii) and 416.926a(e) of this chapter for the definitions of the terms marked and extreme as they apply to children.
3. Rating the limitations of your areas of mental functioning.
a. General. We use all of the relevant medical and non-medical evidence in your case record to evaluate your mental disorder: The symptoms and signs of your disorder, the reported limitations in your activities, and any help and support you receive that is necessary for you to function. The medical evidence may include descriptors regarding the diagnostic stage or level of your disorder, such as “mild” or “moderate.” Clinicians may use these terms to characterize your medical condition. However, these terms will not always be the same as the degree of your limitation in a paragraph B area of mental functioning.
b. Areas of mental functioning in daily activities. You use the same four areas of mental functioning in daily activities at home, at school, and in the community. With respect to a particular task or activity, you may have trouble using one or more of the areas. For example, you may have difficulty understanding and remembering what to do; or concentrating and staying on task long enough to do it; or engaging in the task or activity with other people; or trying to do the task without becoming frustrated and losing self-control. Information about your daily functioning in your activities at home, at school, or in your community can help us understand whether your mental disorder limits one or more of these areas; and, if so, whether it also affects your ability to function age-appropriately.
c. Overall effect of limitations. Limitation of an area of mental functioning reflects the overall degree to which your mental disorder interferes with that area. The degree of limitation does not necessarily reflect a specific type or number of activities, including activities of daily living, that you have difficulty doing. In addition, no single piece of information (including test results) can establish whether you have extreme or marked limitation of an area of mental functioning.
d. Effects of support, supervision, structure on functioning. The degree of limitation of an area of mental functioning also reflects the kind and extent of supports or supervision you receive (beyond what other children your age without impairments typically receive) and the characteristics of any structured setting where you spend your time, which enable you to function. The more extensive the support you need from others (beyond what is age-appropriate) or the more structured the setting you need in order to function, the more limited we will find you to be (see 112.00D).
e. Specific instructions for paragraphs B1, B3, and B4. For paragraphs B1, B3, and B4, the greatest degree of limitation of any part of the area of mental functioning directs the rating of limitation of that whole area of mental functioning.
(i) To do an age-appropriate activity, you must be able to understand and remember and apply information required by the activity. Similarly, you must be able to concentrate and persist and maintain pace in order to complete the activity, and adapt and manage yourself age-appropriately. Limitation in any one of these parts (understand or remember or apply; concentrate or persist or maintain pace; adapt or manage oneself) may prevent you from completing age-appropriate activities.
(ii) We will document the rating of limitation of the whole area of mental functioning, not each individual part. We will not add ratings of the parts together. For example, with respect to paragraph B3, if you have marked limitation in concentrating, but your limitations in persisting and maintaining pace do not rise to a marked level, we will find that you have marked limitation in the whole paragraph B3 area of mental functioning.
(iii) Marked limitation in more than one part of the same paragraph B area of mental functioning does not satisfy the requirement to have marked limitation in two paragraph B areas of mental functioning.
4. How we evaluate mental disorders involving exacerbations and remissions.
a. When we evaluate the effects of your mental disorder, we will consider how often you have exacerbations and remissions, how long they last, what causes your mental disorder to worsen or improve, and any other relevant information. We will assess whether your mental impairment(s) causes marked or extreme limitation of the affected paragraph B area(s) of mental functioning (see 112.00F2). We will consider whether you can use the area of mental functioning age-appropriately on a sustained basis. We will not find that you function age-appropriately solely because you have a period(s) of improvement (remission), or that you are disabled solely because you have a period of worsening (exacerbation), of your mental disorder.
b. If you have a mental disorder involving exacerbations and remissions, you may be able to use the four areas of mental functioning at home, at school, or in the community for a few weeks or months. Recurrence or worsening of symptoms and signs, however, can interfere enough to render you unable to function age-appropriately.
G. What are the paragraph C criteria, and how do we use them to evaluate mental disorders in children age 3 to the attainment of age 18?
1. General. The paragraph C criteria are an alternative to the paragraph B criteria under listings 112.02, 112.03, 112.04, 112.06, and 112.15. We use the paragraph C criteria to evaluate mental disorders that are “serious and persistent.” In the paragraph C criteria, we recognize that mental health interventions may control the more obvious symptoms and signs of your mental disorder.
2. Paragraph C criteria.
a. We find a mental disorder to be “serious and persistent” when there is a medically documented history of the existence of the mental disorder in the listing category over a period of at least 2 years, and evidence shows that your disorder satisfies both C1 and C2.
b. The criterion in C1 is satisfied when the evidence shows that you rely, on an ongoing basis, upon medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s), to diminish the symptoms and signs of your mental disorder (see 112.00D). We consider that you receive ongoing medical treatment when the medical evidence establishes that you obtain medical treatment with a frequency consistent with accepted medical practice for the type of treatment or evaluation required for your medical condition. We will consider periods of inconsistent treatment or lack of compliance with treatment that may result from your mental disorder. If the evidence indicates that the inconsistent treatment or lack of compliance is a feature of your mental disorder, and it has led to an exacerbation of your symptoms and signs, we will not use it as evidence to support a finding that you have not received ongoing medical treatment as required by this paragraph.
c. The criterion in C2 is satisfied when the evidence shows that, despite your diminished symptoms and signs, you have achieved only marginal adjustment. “Marginal adjustment” means that your adaptation to the requirements of daily life is fragile; that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life. We will consider that you have achieved only marginal adjustment when the evidence shows that changes or increased demands have led to exacerbation of your symptoms and signs and to deterioration in your functioning; for example, you have become unable to function outside of your home or a more restrictive setting, without substantial psychosocial supports (see 112.00D). Such deterioration may have necessitated a significant change in medication or other treatment. Similarly, because of the nature of your mental disorder, evidence may document episodes of deterioration that have required you to be hospitalized or absent from school, making it difficult for you to sustain age-appropriate activity over time.
H. How do we document and evaluate intellectual disorder under 112.05?
1. General. Listing 112.05 is based on the two elements that characterize intellectual disorder for children up to age 18: Significantly subaverage general intellectual functioning and significant deficits in current adaptive functioning.
2. Establishing significantly subaverage general intellectual functioning.
a. Definition. Intellectual functioning refers to the general mental capacity to learn, reason, plan, solve problems, and perform other cognitive functions. Under 112.05A, we identify significantly subaverage general intellectual functioning by the cognitive inability to function at a level required to participate in standardized intelligence testing. Our findings under 112.05A are based on evidence from an acceptable medical source. Under 112.05B, we identify significantly subaverage general intellectual functioning by an IQ score(s) on an individually administered standardized test of general intelligence that meets program requirements and has a mean of 100 and a standard deviation of 15. A qualified specialist (see 112.00H2c) must administer the standardized intelligence testing.
b. Psychometric standards. We will find standardized intelligence test results usable for the purposes of 112.05B1 when the measure employed meets contemporary psychometric standards for validity, reliability, normative data, and scope of measurement; and a qualified specialist has individually administered the test according to all pre-requisite testing conditions.
c. Qualified specialist. A “qualified specialist” is currently licensed or certified at the independent level of practice in the State where the test was performed, and has the training and experience to administer, score, and interpret intelligence tests. If a psychological assistant or paraprofessional administered the test, a supervisory qualified specialist must interpret the test findings and co-sign the examination report.
d. Responsibility for conclusions based on testing. We generally presume that your obtained IQ score(s) is an accurate reflection of your general intellectual functioning, unless evidence in the record suggests otherwise. Examples of this evidence include: A statement from the test administrator indicating that your obtained score is not an accurate reflection of your general intellectual functioning, prior or internally inconsistent IQ scores, or information about your daily functioning. Only qualified specialists, Federal and State agency medical and psychological consultants, and other contracted medical and psychological experts may conclude that your obtained IQ score(s) is not an accurate reflection of your general intellectual functioning. This conclusion must be well supported by appropriate clinical and laboratory diagnostic techniques and must be based on relevant evidence in the case record, such as:
(i) The data obtained in testing;
(ii) Your developmental history, including when your signs and symptoms began;
(iii) Information about how you function on a daily basis in a variety of settings; and
(iv) Clinical observations made during the testing period, such as your ability to sustain attention, concentration, and effort; to relate appropriately to the examiner; and to perform tasks independently without prompts or reminders.
3. Establishing significant deficits in adaptive functioning.
a. Definition. Adaptive functioning refers to how you learn and use conceptual, social, and practical skills in dealing with common life demands. It is your typical functioning at home, at school, and in the community, alone or among others. Under 112.05A, we identify significant deficits in adaptive functioning based on your dependence on others to care for your personal needs, such as eating and bathing (grossly in excess of age-appropriate dependence). We will base our conclusions about your adaptive functioning on evidence from a variety of sources (see 112.00H3b) and not on your statements alone. Under 112.05B2, we identify significant deficits in adaptive functioning based on whether there is extreme limitation of one, or marked limitation of two, of the paragraph B criteria (see 112.00E; 112.00F).
b. Evidence. Evidence about your adaptive functioning may come from:
(i) Medical sources, including their clinical observations;
(ii) Standardized tests of adaptive functioning (see 112.00H3c);
(iii) Third party information, such as a report of your functioning from a family member or your caregiver;
(iv) School records;
(v) A teacher questionnaire;
(vi) Reports from employers or supervisors; and
(vii) Your own statements about how you handle all of your daily activities.
c. Standardized tests of adaptive functioning. We do not require the results of an individually administered standardized test of adaptive functioning. If your case record includes these test results, we will consider the results along with all other relevant evidence; however, we will use the guidelines in 112.00E and F to evaluate and determine the degree of your deficits in adaptive functioning, as required under 112.05B2.
d. Standardized developmental assessments. We do not require the results of standardized developmental assessments, which compare your level of development to the level typically expected for your chronological age. If your case record includes test results, we will consider the results along with all other relevant evidence. However, we will use the guidelines in 112.00E and F to evaluate and determine the degree of your deficits in adaptive functioning, as required under 112.05B2.
e. How we consider common everyday activities.
(i) The fact that you engage in common everyday activities, such as caring for your personal needs, preparing simple meals, or driving a car, will not always mean that you do not have deficits in adaptive functioning as required by 112.05B2. You may demonstrate both strengths and deficits in your adaptive functioning. However, a lack of deficits in one area does not negate the presence of deficits in another area. When we assess your adaptive functioning, we will consider all of your activities and your performance of them.
(ii) Our conclusions about your adaptive functioning rest on the quality of your daily activities and whether you do them age-appropriately. If you receive help in performing your activities, we need to know the kind, extent, and frequency of help you receive in order to perform them. We will not assume that your ability to do some common everyday activities, or to do some things without help or support, demonstrates that your mental disorder does not meet the requirements of 112.05B2. (See 112.00D regarding the factors we consider when we evaluate your functioning, including how we consider any help or support you receive.)
f. How we consider work activity. The fact that you have engaged in work activity, or that you work intermittently or steadily in a job commensurate with your abilities, will not always mean that you do not have deficits in adaptive functioning as required by 112.05B2. When you have engaged in work activity, we need complete information about the work, and about your functioning in the work activity and work setting, before we reach any conclusions about your adaptive functioning. We will consider all factors involved in your work history before concluding whether your impairment satisfies the criteria for intellectual disorder under 112.05B. We will consider your prior and current work history, if any, and various other factors influencing how you function. For example, we consider whether the work was in a supported setting, whether you required more supervision than other employees, how your job duties compared to others in the same job, how much time it took you to learn the job duties, and the reason the work ended, if applicable.
I. What additional considerations do we use to evaluate developmental disorders of infants and toddlers?
1. General. We evaluate developmental disorders from birth to attainment of age 3 under 112.14. We evaluate your ability to acquire and maintain the motor, cognitive, social/communicative, and emotional skills that you need to function age-appropriately. When we rate your impairment-related limitations for this listing (see §§ 416.925(b)(2)(ii) and 416.926a(e) of this chapter), we consider only limitations you have because of your developmental disorder. If you have a chronic illness or physical abnormality(ies), we will evaluate it under the affected body system, for example, the cardiovascular or musculoskeletal system.
2. Age and typical development in early childhood.
a. Prematurity and age. If you were born prematurely, we will use your corrected chronological age (CCA) for comparison. CCA is your chronological age adjusted by a period of gestational prematurity. CCA = (chronological age)−(number of weeks premature). If you have not attained age 1, we will correct your chronological age, using the same formula. If you are over age 1, we will decide whether to correct your chronological age, based on our judgment and all the facts of your case (see § 416.924b(b) of this chapter).
b. Developmental assessment. We will use the results from a standardized developmental assessment to compare your level of development with that typically expected for your chronological age. When there are no results from a comprehensive standardized developmental assessment in the case record, we need narrative developmental reports from your medical sources in sufficient detail to assess the limitations resulting from your developmental disorder.
c. Variation. When we evaluate your developmental disorder, we will consider the wide variation in the range of normal or typical development in early childhood. At the end of a recognized milestone period, new skills typically begin to emerge. If your new skills begin to emerge later than is typically expected, the timing of their emergence may or may not indicate that you have a developmental delay or deficit that can be expected to last for 1 year.
3. Evidence.
a. Standardized developmental assessments. We use standardized test reports from acceptable medical sources or from early intervention specialists, physical or occupational therapists, and other qualified professionals. Only the qualified professional who administers the test, Federal and State agency medical and psychological consultants, and other contracted medical and psychological experts may conclude that the assessment results are not an accurate reflection of your development. This conclusion must be well supported by appropriate clinical and laboratory diagnostic techniques and must be based on relevant evidence in the case record. If the assessment results are not an accurate reflection of your development, we may purchase a new developmental assessment. If the developmental assessment is inconsistent with other information in your case record, we will follow the guidelines in § 416.920b of this chapter.
b. Narrative developmental reports. A narrative developmental report is based on clinical observations, progress notes, and well-baby check-ups, and includes your developmental history, examination findings (with abnormal findings noted on repeated examinations), and an overall assessment of your development (that is, more than one or two isolated skills) by the medical source. Although medical sources may refer to screening test results as supporting evidence in the narrative developmental report, screening test results alone cannot establish a diagnosis or the severity of developmental disorder.
4. What are the paragraph B criteria for 112.14?
a. General. The paragraph B criteria for 112.14 are slightly different from the paragraph B criteria for the other listings. They are the developmental abilities that infants and toddlers use to acquire and maintain the skills needed to function age-appropriately. An infant or toddler is expected to use his or her developmental abilities to achieve a recognized pattern of milestones, over a typical range of time, in order to acquire and maintain the skills needed to function age-appropriately. We will find that your developmental disorder satisfies the requirements of 112.14 if it results in extreme limitation of one, or marked limitation of two, of the 112.14 paragraph B criteria. (See §§ 416.925(b)(2)(ii) and 416.926a(e) of this chapter for the definitions of the terms marked and extreme as they apply to children.)
b. Definitions of the 112.14 paragraph B developmental abilities.
(i) Ability to plan and control motor movement. This criterion refers to the developmental ability to plan, remember, and execute controlled motor movements by integrating and coordinating perceptual and sensory input with motor output. Using this ability develops gross and fine motor skills, and makes it possible for you to engage in age-appropriate symmetrical or alternating motor activities. You use this ability when, for example, you grasp and hold objects with one or both hands, pull yourself up to stand, walk without holding on, and go up and down stairs with alternating feet. These examples illustrate the nature of the developmental ability. We do not require documentation of all of the examples. How you manifest this developmental ability and your limitations in using it depends, in part, on your age.
(ii) Ability to learn and remember. This criterion refers to the developmental ability to learn by exploring the environment, engaging in trial-and-error experimentation, putting things in groups, understanding that words represent things, and participating in pretend play. Using this ability develops the skills that help you understand what things mean, how things work, and how you can make things happen. You use this ability when, for example, you show interest in objects that are new to you, imitate simple actions, name body parts, understand simple cause-and-effect relationships, remember simple directions, or figure out how to take something apart. These examples illustrate the nature of the developmental ability. We do not require documentation of all of the examples. How you manifest this developmental ability and your limitations in using it depends, in part, on your age.
(iii) Ability to interact with others. This criterion refers to the developmental ability to participate in reciprocal social interactions and relationships by communicating your feelings and intents through vocal and visual signals and exchanges; physical gestures and contact; shared attention and affection; verbal turn taking; and understanding and sending increasingly complex messages. Using this ability develops the social skills that make it possible for you to influence others (for example, by gesturing for a toy or saying “no” to stop an action); invite someone to interact with you (for example, by smiling or reaching); and draw someone's attention to what interests you (for example, by pointing or taking your caregiver's hand and leading that person). You use this ability when, for example, you use vocalizations to initiate and sustain a “conversation” with your caregiver; respond to limits set by an adult with words, gestures, or facial expressions; play alongside another child; or participate in simple group activities with adult help. These examples illustrate the nature of the developmental ability. We do not require documentation of all of the examples. How you manifest this developmental ability and your limitations in using it depends, in part, on your age.
(iv) Ability to regulate physiological functions, attention, emotion, and behavior. This criterion refers to the developmental ability to stabilize biological rhythms (for example, by developing an age-appropriate sleep/wake cycle); control physiological functions (for example, by achieving regular patterns of feeding); and attend, react, and adapt to environmental stimuli, persons, objects, and events (for example, by becoming alert to things happening around you and in relation to you, and responding without overreacting or underreacting). Using this ability develops the skills you need to regulate yourself and makes it possible for you to achieve and maintain a calm, alert, and organized physical and emotional state. You use this ability when, for example, you recognize your body's needs for food or sleep, focus quickly and pay attention to things that interest you, cry when you are hurt but become quiet when your caregiver holds you, comfort yourself with your favorite toy when you are upset, ask for help when something frustrates you, or refuse help from your caregiver when trying to do something for yourself. These examples illustrate the nature of the developmental ability. We do not require documentation of all of the examples. How you manifest this developmental ability and your limitations in using it depends, in part, on your age.
5. Deferral of determination.
a. Full-term infants. In the first few months of life, full-term infants typically display some irregularities in observable behaviors (for example, sleep cycles, feeding, responding to stimuli, attending to faces, self-calming), making it difficult to assess the presence, extent, and duration of a developmental disorder. When the evidence indicates that you may have a significant developmental delay, but there is insufficient evidence to make a determination, we will defer making a disability determination under 112.14 until you are at least 6 months old. This deferral will allow us to obtain a longitudinal medical history so that we can more accurately evaluate your developmental patterns and functioning over time. In most cases, when you are at least 6 months old, any developmental delay you may have can be better assessed, and you can undergo standardized developmental testing, if indicated.
b. Premature infants. When the evidence indicates that you may have a significant developmental delay, but there is insufficient evidence to make a determination, we will defer your case until you attain a CCA (see 112.00I2a) of at least 6 months in order to better evaluate your developmental delay.
c. When we will not defer a determination. We will not defer our determination if we have sufficient evidence to determine that you are disabled under 112.14 or any other listing, or that you have an impairment or combination of impairments that functionally equals the listings. In addition, we will not defer our determination if the evidence demonstrates that you are not disabled.
J. How do we evaluate substance use disorders? If we find that you are disabled and there is medical evidence in your case record establishing that you have a substance use disorder, we will determine whether your substance use disorder is a contributing factor material to the determination of disability (see § 416.935 of this chapter).
K. How do we evaluate mental disorders that do not meet one of the mental disorders listings?
1. These listings include only examples of mental disorders that we consider serious enough to result in marked and severe functional limitations. If your severe mental disorder does not meet the criteria of any of these listings, we will consider whether you have an impairment(s) that meets the criteria of a listing in another body system. You may have another impairment(s) that is secondary to your mental disorder. For example, if you have an eating disorder and develop a cardiovascular impairment because of it, we will evaluate your cardiovascular impairment under the listings for the cardiovascular body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing (see § 416.926 of this chapter).
3. If your impairment(s) does not meet or medically equal a listing, we will consider whether you have an impairment(s) that functionally equals the listings (see § 416.926a of this chapter).
4. Although we present these alternatives in a specific sequence above, each represents listing-level severity, and we can evaluate your claim in any order. For example, if the factors of your case indicate that the combination of your impairments may functionally equal the listings, we may start with that analysis. We use the rules in § 416.994a of this chapter, as appropriate, when we decide whether you continue to be disabled.
112.01 Category of Impairments, Mental Disorders112.02 Neurocognitive disorders (see 112.00B1), for children age 3 to attainment of age 18, satisfied by A and B, or A and C:
A. Medical documentation of a clinically significant deviation in normal cognitive development or by significant cognitive decline from a prior level of functioning in one or more of the cognitive areas:
1. Complex attention;
2. Executive function;
3. Learning and memory;
4. Language;
5. Perceptual-motor; or
6. Social cognition.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 112.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 112.00G2c).
112.03 Schizophrenia spectrum and other psychotic disorders (see 112.00B2), for children age 3 to attainment of age 18, satisfied by A and B, or A and C:
A. Medical documentation of one or more of the following:
1. Delusions or hallucinations;
2. Disorganized thinking (speech); or
3. Grossly disorganized behavior or catatonia.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 112.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 112.00G2c).
112.04 Depressive, bipolar and related disorders (see 112.00B3), for children age 3 to attainment of age 18, satisfied by A and B, or A and C:
A. Medical documentation of the requirements of paragraph 1, 2, or 3:
1. Depressive disorder, characterized by five or more of the following:
a. Depressed or irritable mood;
b. Diminished interest in almost all activities;
c. Appetite disturbance with change in weight (or a failure to achieve an expected weight gain);
d. Sleep disturbance;
e. Observable psychomotor agitation or retardation;
f. Decreased energy;
g. Feelings of guilt or worthlessness;
h. Difficulty concentrating or thinking; or
i. Thoughts of death or suicide.
2. Bipolar disorder, characterized by three or more of the following:
a. Pressured speech;
b. Flight of ideas;
c. Inflated self-esteem;
d. Decreased need for sleep;
e. Distractibility;
f. Involvement in activities that have a high probability of painful consequences that are not recognized; or
g. Increase in goal-directed activity or psychomotor agitation.
3. Disruptive mood dysregulation disorder, beginning prior to age 10, and all of the following:
a. Persistent, significant irritability or anger;
b. Frequent, developmentally inconsistent temper outbursts; and
c. Frequent aggressive or destructive behavior.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 112.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 112.00G2c).
112.05 Intellectual disorder (see 112.00B4), for children age 3 to attainment of age 18, satisfied by A or B:
A. Satisfied by 1 and 2 (see 112.00H):
1. Significantly subaverage general intellectual functioning evident in your cognitive inability to function at a level required to participate in standardized testing of intellectual functioning; and
2. Significant deficits in adaptive functioning currently manifested by your dependence upon others for personal needs (for example, toileting, eating, dressing, or bathing) in excess of age-appropriate dependence.
ORB. Satisfied by 1 and 2 (see 112.00H):
1. Significantly subaverage general intellectual functioning evidenced by a or b:
a. A full scale (or comparable) IQ score of 70 or below on an individually administered standardized test of general intelligence; or
b. A full scale (or comparable) IQ score of 71-75 accompanied by a verbal or performance IQ score (or comparable part score) of 70 or below on an individually administered standardized test of general intelligence; and
2. Significant deficits in adaptive functioning currently manifested by extreme limitation of one, or marked limitation of two, of the following areas of mental functioning:
a. Understand, remember, or apply information (see 112.00E1); or
b. Interact with others (see 112.00E2); or
c. Concentrate, persist, or maintain pace (see 112.00E3); or
d. Adapt or manage oneself (see 112.00E4).
112.06 Anxiety and obsessive-compulsive disorders (see 112.00B5), for children age 3 to attainment of age 18, satisfied by A and B, or A and C:
A. Medical documentation of the requirements of paragraph 1, 2, 3, or 4:
1. Anxiety disorder, characterized by one or more of the following:
a. Restlessness;
b. Easily fatigued;
c. Difficulty concentrating;
d. Irritability;
e. Muscle tension; or
f. Sleep disturbance.
2. Panic disorder or agoraphobia, characterized by one or both:
a. Panic attacks followed by a persistent concern or worry about additional panic attacks or their consequences; or
b. Disproportionate fear or anxiety about at least two different situations (for example, using public transportation, being in a crowd, being in a line, being outside of your home, being in open spaces).
3. Obsessive-compulsive disorder, characterized by one or both:
a. Involuntary, time-consuming preoccupation with intrusive, unwanted thoughts; or;
b. Repetitive behaviors that appear aimed at reducing anxiety.
4. Excessive fear or anxiety concerning separation from those to whom you are attached.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 112.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 112.00G2c).
112.07 Somatic symptom and related disorders (see 112.00B6), for children age 3 to attainment of age 18, satisfied by A and B:
A. Medical documentation of one or both of the following:
1. Symptoms of altered voluntary motor or sensory function that are not better explained by another medical or mental disorder; or
2. One or more somatic symptoms that are distressing, with excessive thoughts, feelings, or behaviors related to the symptoms.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
112.08 Personality and impulse-control disorders (see 112.00B7), for children age 3 to attainment of age 18, satisfied by A and B:
A. Medical documentation of a pervasive pattern of one or more of the following:
1. Distrust and suspiciousness of others;
2. Detachment from social relationships;
3. Disregard for and violation of the rights of others;
4. Instability of interpersonal relationships;
5. Excessive emotionality and attention seeking;
6. Feelings of inadequacy;
7. Excessive need to be taken care of;
8. Preoccupation with perfectionism and orderliness; or
9. Recurrent, impulsive, aggressive behavioral outbursts.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
112.09 [Reserved]
112.10 Autism spectrum disorder (see 112.00B8), for children age 3 to attainment of age 18), satisfied by A and B:
A. Medical documentation of both of the following:
1. Qualitative deficits in verbal communication, nonverbal communication, and social interaction; and
2. Significantly restricted, repetitive patterns of behavior, interests, or activities.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
112.11 Neurodevelopmental disorders (see 112.00B9), for children age 3 to attainment of age 18, satisfied by A and B:
A. Medical documentation of the requirements of paragraph 1, 2, or 3:
1. One or both of the following:
a. Frequent distractibility, difficulty sustaining attention, and difficulty organizing tasks; or
b. Hyperactive and impulsive behavior (for example, difficulty remaining seated, talking excessively, difficulty waiting, appearing restless, or behaving as if being “driven by a motor”).
2. Significant difficulties learning and using academic skills; or
3. Recurrent motor movement or vocalization.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
112.12 [Reserved]
112.13 Eating disorders (see 112.00B10), for children age 3 to attainment of age 18, satisfied by A and B:
A. Medical documentation of a persistent alteration in eating or eating-related behavior that results in a change in consumption or absorption of food and that significantly impairs physical or psychological health.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
112.14 Developmental disorders in infants and toddlers (see 112.00B11, 112.00I), satisfied by A and B:
A. Medical documentation of one or both of the following:
1. A delay or deficit in the development of age-appropriate skills; or
2. A loss of previously acquired skills.
ANDB. Extreme limitation of one, or marked limitation of two, of the following developmental abilities (see 112.00F):
1. Plan and control motor movement (see 112.00I4b(i)).
2. Learn and remember (see 112.00I4b(ii)).
3. Interact with others (see 112.00I4b(iii)).
4. Regulate physiological functions, attention, emotion, and behavior (see 112.00I4b(iv)).
112.15 Trauma- and stressor-related disorders (see 112.00B11), for children age 3 to attainment of age 18, satisfied by A and B, or A and C:
A. Medical documentation of the requirements of paragraph 1 or 2:
1. Posttraumatic stress disorder, characterized by all of the following:
a. Exposure to actual or threatened death, serious injury, or violence;
b. Subsequent involuntary re-experiencing of the traumatic event (for example, intrusive memories, dreams, or flashbacks);
c. Avoidance of external reminders of the event;
d. Disturbance in mood and behavior (for example, developmental regression, socially withdrawn behavior); and
e. Increases in arousal and reactivity (for example, exaggerated startle response, sleep disturbance).
2. Reactive attachment disorder, characterized by two or all of the following:
a. Rarely seeks comfort when distressed;
b. Rarely responds to comfort when distressed; or
c. Episodes of unexplained emotional distress.
ANDB. Extreme limitation of one, or marked limitation of two, of the following areas of mental functioning (see 112.00F):
1. Understand, remember, or apply information (see 112.00E1).
2. Interact with others (see 112.00E2).
3. Concentrate, persist, or maintain pace (see 112.00E3).
4. Adapt or manage oneself (see 112.00E4).
ORC. Your mental disorder in this listing category is “serious and persistent;” that is, you have a medically documented history of the existence of the disorder over a period of at least 2 years, and there is evidence of both:
1. Medical treatment, mental health therapy, psychosocial support(s), or a highly structured setting(s) that is ongoing and that diminishes the symptoms and signs of your mental disorder (see 112.00G2b); and
2. Marginal adjustment, that is, you have minimal capacity to adapt to changes in your environment or to demands that are not already part of your daily life (see 112.00G2c).
113.00 Cancer (Malignant Neoplastic Diseases)A. What impairments do these listings cover? We use these listings to evaluate all cancers (malignant neoplastic diseases) except certain cancers associated with human immunodeficiency virus (HIV) infection. We use the criteria in 114.11B to evaluate primary central nervous system lymphoma, 114.11C to evaluate primary effusion lymphoma, and 114.11E to evaluate pulmonary Kaposi sarcoma if you also have HIV infection. We evaluate all other cancers associated with HIV infection, for example, Hodgkin lymphoma or non-pulmonary Kaposi sarcoma, under this body system or under 114.11F-I in the immune system disorders body system.
B. What do we consider when we evaluate cancer under these listings? We will consider factors including:
1. Origin of the cancer.
2. Extent of involvement.
3. Duration, frequency, and response to anticancer therapy.
4. Effects of any post-therapeutic residuals.
C. How do we apply these listings? We apply the criteria in a specific listing to a cancer originating from that specific site.
D. What evidence do we need?
1. We need medical evidence that specifies the type, extent, and site of the primary, recurrent, or metastatic lesion. When the primary site cannot be identified, we will use evidence documenting the site(s) of metastasis to evaluate the impairment under 13.27 in part A.
2. For operative procedures, including a biopsy or a needle aspiration, we generally need a copy of both the:
a. Operative note, and
b. Pathology report.
3. When we cannot get these documents, we will accept the summary of hospitalization(s) or other medical reports. This evidence should include details of the findings at surgery and, whenever appropriate, the pathological findings.
4. In some situations, we may also need evidence about recurrence, persistence, or progression of the cancer, the response to therapy, and any significant residuals. (See 113.00G.)
E. When do we need longitudinal evidence?
1. Cancer with distant metastases. Most cancer of childhood consists of a local lesion with metastases to regional lymph nodes and, less often, distant metastases. We generally do not need longitudinal evidence for cancer that has metastasized beyond the regional lymph nodes because this cancer usually meets the requirements of a listing. Exceptions are for cancer with distant metastases that we expect to respond to anticancer therapy. For these exceptions, we usually need a longitudinal record of 3 months after therapy starts to determine whether the therapy achieved its intended effect, and whether this effect is likely to persist.
2. Other cancers. When there are no distant metastases, many of the listings require that we consider your response to initial anticancer therapy; that is, the initial planned treatment regimen. This therapy may consist of a single modality or a combination of modalities; that is, multimodal therapy (see 113.00I3).
3. Types of treatment.
a. Whenever the initial planned therapy is a single modality, enough time must pass to allow a determination about whether the therapy will achieve its intended effect. If the treatment fails, the failure often happens within 6 months after treatment starts, and there will often be a change in the treatment regimen.
b. Whenever the initial planned therapy is multimodal, we usually cannot make a determination about the effectiveness of the therapy until we can determine the effects of all the planned modalities. In some cases, we may need to defer adjudication until we can assess the effectiveness of therapy. However, we do not need to defer adjudication to determine whether the therapy will achieve its intended effect if we can make a fully favorable determination or decision based on the length and effects of therapy, or the residuals of the cancer or therapy (see 113.00G).
F. How do we evaluate impairments that do not meet one of the cancer listings?
1. These listings are only examples of cancers that we consider severe enough to result in marked and severe functional limitations. If your severe impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that meets the criteria of a listing in another body system.
2. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See §§ 404.1526 and 416.926 of this chapter.) If your impairment(s) does not meet or medically equal a listing, we will also consider whether you have an impairment(s) that functionally equals the listings. (See § 416.926a of this chapter.) We use the rules in § 416.994a of this chapter when we decide whether you continue to be disabled.
G. How do we consider the effects of anticancer therapy?
1. How we consider the effects of anticancer therapy under the listings. In many cases, cancers meet listing criteria only if the therapy is not effective and the cancer persists, progresses, or recurs. However, as explained in the following paragraphs, we will not delay adjudication if we can make a fully favorable determination or decision based on the evidence in the case record.
2. Effects can vary widely.
a. We consider each case on an individual basis because the therapy and its toxicity may vary widely. We will request a specific description of the therapy, including these items:
i. Drugs given.
ii. Dosage.
iii. Frequency of drug administration.
iv. Plans for continued drug administration.
v. Extent of surgery.
vi. Schedule and fields of radiation therapy.
b. We will also request a description of the complications or adverse effects of therapy, such as the following:
i. Continuing gastrointestinal symptoms.
ii. Persistent weakness.
iii. Neurological complications.
iv. Cardiovascular complications.
v. Reactive mental disorders.
3. Effects of therapy may change. The severity of the adverse effects of anticancer therapy may change during treatment; therefore, enough time must pass to allow us to evaluate the therapy's effect. The residual effects of treatment are temporary in most instances; however, on occasion, the effects may be disabling for a consecutive period of at least 12 months. In some situations, very serious adverse effects may interrupt and prolong multimodal anticancer therapy for a continuous period of almost 12 months. In these situations, we may determine there is an expectation that your impairment will preclude you from engaging in any age-appropriate activities for at least 12 months.
4. When the initial anticancer therapy is effective. We evaluate any post-therapeutic residual impairment(s) not included in these listings under the criteria for the affected body system. We must consider any complications of therapy. When the residual impairment(s) does not meet a listing, we must consider whether it medically equals a listing, or, as appropriate, functionally equals the listings.
H. How long do we consider your impairment to be disabling?
1. In some listings, we specify that we will consider your impairment to be disabling until a particular point in time (for example, until at least 12 months from the date of transplantation). We may consider your impairment to be disabling beyond this point when the medical and other evidence justifies it.
2. When a listing does not contain such a specification, we will consider an impairment(s) that meets or medically equals a listing in this body system to be disabling until at least 3 years after onset of complete remission. When the impairment(s) has been in complete remission for at least 3 years, that is, the original tumor or a recurrence (or relapse) and any metastases have not been evident for at least 3 years, the impairment(s) will no longer meet or medically equal the criteria of a listing in this body system.
3. Following the appropriate period, we will consider any residuals, including residuals of the cancer or therapy (see 113.00G), in determining whether you are disabled. If you have a recurrence or relapse of your cancer, your impairment may meet or medically equal one of the listings in this body system again.
I. What do we mean by the following terms?
1. Anticancer therapy means surgery, radiation, chemotherapy, hormones, immunotherapy, or bone marrow or stem cell transplantation. When we refer to surgery as an anticancer treatment, we mean surgical excision for treatment, not for diagnostic purposes.
2. Metastases means the spread of cancer cells by blood, lymph, or other body fluid. This term does not include the spread of cancer cells by direct extension of the cancer to other tissues or organs.
3. Multimodal therapy means anticancer therapy that is a combination of at least two types of treatment given in close proximity as a unified whole and usually planned before any treatment has begun. There are three types of treatment modalities: Surgery, radiation, and systemic drug therapy (chemotherapy, hormone therapy, and immunotherapy or biological modifier therapy). Examples of multimodal therapy include:
a. Surgery followed by chemotherapy or radiation.
b. Chemotherapy followed by surgery.
c. Chemotherapy and concurrent radiation.
4. Persistent means the planned initial anticancer therapy failed to achieve a complete remission of your cancer; that is, your cancer is evident, even if smaller, after the therapy has ended.
5. Progressive means the cancer becomes more extensive after treatment; that is, there is evidence that your cancer is growing after you have completed at least half of your planned initial anticancer therapy.
6. Recurrent or relapse means the cancer that was in complete remission or entirely removed by surgery has returned.
J. Can we establish the existence of a disabling impairment prior to the date of the evidence that shows the cancer satisfies the criteria of a listing? Yes. We will consider factors such as:
1. The type of cancer and its location.
2. The extent of involvement when the cancer was first demonstrated.
3. Your symptoms.
K. How do we evaluate specific cancers?
1. Lymphoma.
a. We provide criteria for evaluating lymphomas that are disseminated or have not responded to anticancer therapy in 113.05.
b. Lymphoblastic lymphoma is treated with leukemia-based protocols, so we evaluate this type of cancer under 113.06.
2. Leukemia.
a. Acute leukemia. The initial diagnosis of acute leukemia, including the accelerated or blast phase of chronic myelogenous (granulocytic) leukemia, is based on definitive bone marrow examination. Additional diagnostic information is based on chromosomal analysis, cytochemical and surface marker studies on the abnormal cells, or other methods consistent with the prevailing state of medical knowledge and clinical practice. Recurrent disease must be documented by peripheral blood, bone marrow, or cerebrospinal fluid examination, or by testicular biopsy. The initial and follow-up pathology reports should be included.
b. Chronic myelogenous leukemia (CML). We need a diagnosis of CML based on documented granulocytosis, including immature forms such as differentiated or undifferentiated myelocytes and myeloblasts, and a chromosomal analysis that demonstrates the Philadelphia chromosome. In the absence of a chromosomal analysis, or if the Philadelphia chromosome is not present, the diagnosis may be made by other methods consistent with the prevailing state of medical knowledge and clinical practice. The requirement for CML in the accelerated or blast phase is met in 113.06B if laboratory findings show the proportion of blast (immature) cells in the peripheral blood or bone marrow is 10 percent or greater.
c. Juvenile chronic myelogenous leukemia (JCML). JCML is a rare, Philadelphia-chromosome-negative childhood leukemia that is aggressive and clinically similar to acute myelogenous leukemia. We evaluate JCML under 113.06A.
d. Elevated white cell count. In cases of chronic leukemia (either myelogenous or lymphocytic), an elevated white cell count, in itself, is not a factor in determining the severity of the impairment.
3. Malignant solid tumors. The tumors we consider under 113.03 include the histiocytosis syndromes except for solitary eosinophilic granuloma. We do not evaluate thyroid cancer (see 113.09), retinoblastomas (see 113.12), primary central nervous system (CNS) cancers (see 113.13), neuroblastomas (see 113.21), or malignant melanoma (see 113.29) under this listing.
4. Primary central nervous system (CNS) cancers. We use the criteria in 113.13 to evaluate cancers that originate within the CNS (that is, brain and spinal cord cancers).
a. The CNS cancers listed in 113.13A are highly malignant and respond poorly to treatment, and therefore we do not require additional criteria to evaluate them. We do not list pituitary gland cancer (for example, pituitary gland carcinoma) in 113.13A, although this CNS cancer is highly malignant and responds poorly to treatment. We evaluate pituitary gland cancer under 113.13A and do not require additional criteria to evaluate it.
b. We consider a CNS tumor to be malignant if it is classified as Grade II, Grade III, or Grade IV under the World Health Organization (WHO) classification of tumors of the CNS (WHO Classification of Tumours of the Central Nervous System, 2007).
c. We evaluate benign (for example, WHO Grade I) CNS tumors under 111.05. We evaluate metastasized CNS cancers from non-CNS sites under the primary cancers (see 113.00C). We evaluate any complications of CNS cancers, such as resultant neurological or psychological impairments, under the criteria for the affected body system.
5. Retinoblastoma. The treatment for bilateral retinoblastoma usually results in a visual impairment. We will evaluate any resulting visual impairment under 102.02.
6. Melanoma. We evaluate malignant melanoma that affects the skin (cutaneous melanoma), eye (ocular melanoma), or mucosal membranes (mucosal melanoma) under 113.29. We evaluate melanoma that is not malignant that affects the skin (benign melanocytic tumor) under the listings in 108.00 or other affected body systems.
L. How do we evaluate cancer treated by bone marrow or stem cell transplantation, including transplantation using stem cells from umbilical cord blood? Bone marrow or stem cell transplantation is performed for a variety of cancers. We require the transplantation to occur before we evaluate it under these listings. We do not need to restrict our determination of the onset of disability to the date of transplantation (113.05 or 113.06). We may be able to establish an earlier onset date of disability due to your transplantation if the evidence in your case record supports such a finding.
1. Acute leukemia (including all types of lymphoblastic lymphomas and JCML) or accelerated or blast phase of CML. If you undergo bone marrow or stem cell transplantation for any of these disorders, we will consider you to be disabled until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of transplantation, whichever is later.
2. Lymphoma or chronic phase of CML. If you undergo bone marrow or stem cell transplantation for any of these disorders, we will consider you to be disabled until at least 12 months from the date of transplantation.
3. Evaluating disability after the appropriate time period has elapsed. We consider any residual impairment(s), such as complications arising from:
a. Graft-versus-host (GVH) disease.
b. Immunosuppressant therapy, such as frequent infections.
c. Significant deterioration of other organ systems.
113.01 Category of Impairments, Cancer (Malignant Neoplastic Diseases)
113.01 Category of Impairments, Malignant Neoplastic Diseases113.03 Malignant solid tumors. Consider under a disability:
A. For 24 months from the date of initial diagnosis. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
ORB. For 24 months from the date of recurrence of active disease. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
113.05 Lymphoma (excluding all types of lymphoblastic lymphomas - 113.06). (See 113.00K1.)
A. Non-Hodgkin lymphoma (including Burkitt's and anaplastic large cell), with either 1 or 2:
1. Bone marrow, brain, spinal cord, liver, or lung involvement at initial diagnosis. Consider under a disability for 24 months from the date of diagnosis. Thereafter, evaluate under 113.05A2, or any residual impairments(s) under the criteria for the affected body system.
2. Persistent or recurrent following initial anticancer therapy.
ORB. Hodgkin lymphoma, with either 1 or 2:
1. Bone marrow, brain, spinal cord, liver, or lung involvement at initial diagnosis. Consider under a disability for 24 months from the date of diagnosis. Thereafter, evaluate under 113.05B2, or any residual impairment(s) under the criteria for the affected body system.
2. Persistent or recurrent following initial anticancer therapy.
ORC. With bone marrow or stem cell transplantation. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria of the affected body system.
ORD. Mantle cell lymphoma.
113.06 Leukemia. (See 113.00K2.)
A. Acute leukemia (including all types of lymphoblastic lymphomas and juvenile chronic myelogenous leukemia (JCML)). Consider under a disability until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of bone marrow or stem cell transplantation, whichever is later. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
ORB. Chronic myelogenous leukemia (except JCML), as described in 1 or 2:
1. Accelerated or blast phase (see 113.00K2b). Consider under a disability until at least 24 months from the date of diagnosis or relapse, or at least 12 months from the date of bone marrow or stem cell transplantation, whichever is later. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
2. Chronic phase, as described in a or b:
a. Consider under a disability until at least 12 months from the date of bone marrow or stem cell transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
b. Progressive disease following initial antineoplastic therapy.
113.09 Thyroid gland.
A. Anaplastic (undifferentiated) carcinoma.
ORB. Carcinoma with metastases beyond the regional lymph nodes progressive despite radioactive iodine therapy.
ORC. Medullary carcinoma with metastases beyond the regional lymph nodes.
113.12 Retinoblastoma.
A. With extension beyond the orbit.
ORB. Persistent or recurrent following initial anticancer therapy.
ORC. With regional or distant metastases.
113.13 Nervous system. (See 113.00K4.) Primary central nervous system (CNS; that is, brain and spinal cord) cancers, as described in A, B, or C:
A. Glioblastoma multiforme, ependymoblastoma, and diffuse intrinsic brain stem gliomas (see 113.00K4a).
B. Any Grade III or Grade IV CNS cancer (see 113.00K4b), including astrocytomas, sarcomas, and medulloblastoma and other primitive neuroectodermal tumors (PNETs).
C. Any primary CNS cancer, as described in 1 or 2:
1. Metastatic.
2. Progressive or recurrent following initial anticancer therapy.
113.21 Neuroblastoma.
A. With extension across the midline.
ORB. With distant metastases.
ORC. Recurrent.
ORD. With onset at age 1 year or older.
113.29 Malignant melanoma (including skin, ocular, or mucosal melanomas), as described in either A, B, or C:
A. Recurrent (except an additional primary melanoma at a different site, which is not considered to be recurrent disease) following either 1 or 2:
1. Wide excision (skin melanoma).
2. Enucleation of the eye (ocular melanoma).
ORB. With metastases as described in 1, 2, or 3:
1. Metastases to one or more clinically apparent nodes; that is, nodes that are detected by imaging studies (excluding lymphoscintigraphy) or by clinical evaluation (palpable).
2. If the nodes are not clinically apparent, with metastases to four or more nodes.
3. Metastases to adjacent skin (satellite lesions) or distant sites (for example, liver, lung, or brain).
ORC. Mucosal melanoma.
114.00 Immune System Disorders A. What disorders do we evaluate under the immune system disorders listings?1. We evaluate immune system disorders that cause dysfunction in one or more components of your immune system.
a. The dysfunction may be due to problems in antibody production, impaired cell-mediated immunity, a combined type of antibody/cellular deficiency, impaired phagocytosis, or complement deficiency.
b. Immune system disorders may result in recurrent and unusual infections, or inflammation and dysfunction of the body's own tissues. Immune system disorders can cause a deficit in a single organ or body system that results in extreme (that is, very serious) loss of function. They can also cause lesser degrees of limitations in two or more organs or body systems, and when associated with symptoms or signs, such as severe fatigue, fever, malaise, diffuse musculoskeletal pain, or involuntary weight loss, can also result in extreme limitation. In children, immune system disorders or their treatment may also affect growth, development, and the performance of age-appropriate activities.
c. We organize the discussions of immune system disorders in three categories: Autoimmune disorders; Immune deficiency disorders, excluding human immunodeficiency virus (HIV) infection; and HIV infection.
2. Autoimmune disorders (114.00D). Autoimmune disorders are caused by dysfunctional immune responses directed against the body's own tissues, resulting in chronic, multisystem impairments that differ in clinical manifestations, course, and outcome. They are sometimes referred to as rheumatic diseases, connective tissue disorders, or collagen vascular disorders. Some of the features of autoimmune disorders in children differ from the features of the same disorders in adults. The impact of the disorders or their treatment on physical, psychological, and developmental growth of pre-pubertal children may be considerable, and often differs from that of post-pubertal adolescents or adults.
3. Immune deficiency disorders, excluding HIV infection (114.00E). Immune deficiency disorders are characterized by recurrent or unusual infections that respond poorly to treatment, and are often associated with complications affecting other parts of the body. Immune deficiency disorders are classified as either primary (congenital) or acquired. Children with immune deficiency disorders also have an increased risk of malignancies and of having autoimmune disorders.
4. Human immunodeficiency virus (HIV) infection (114.00F). HIV infection may be characterized by increased susceptibility to common infections as well as opportunistic infections, cancers, or other conditions listed in 114.11.
B. What information do we need to show that you have an immune system disorder?Generally, we need your medical history, a report(s) of a physical examination, a report(s) of laboratory findings, and in some instances, appropriate medically acceptable imaging or tissue biopsy reports to show that you have an immune system disorder. Therefore, we will make every reasonable effort to obtain your medical history, medical findings, and results of laboratory tests. We explain the information we need in more detail in the sections below.
C. Definitions1. Appropriate medically acceptable imaging includes, but is not limited to, angiography, x-ray imaging, computerized axial tomography (CAT scan) or magnetic resonance imaging (MRI), with or without contrast material, myelography, and radionuclear bone scans. “Appropriate” means that the technique used is the proper one to support the evaluation and diagnosis of the impairment.
2. Constitutional symptoms or signs, as used in these listings, means severe fatigue, fever, malaise, or involuntary weight loss. Severe fatigue means a frequent sense of exhaustion that results in significantly reduced physical activity or mental function. Malaise means frequent feelings of illness, bodily discomfort, or lack of well-being that result in significantly reduced physical activity or mental function.
3. Disseminated means that a condition is spread over a considerable area. The type and extent of the spread will depend on your specific disease.
4. Dysfunction means that one or more of the body regulatory mechanisms are impaired, causing either an excess or deficiency of immunocompetent cells or their products.
5. Extra-articular means “other than the joints”; for example, an organ(s) such as the heart, lungs, kidneys, or skin.
6. Inability to ambulate effectively has the same meaning as in 101.00B2b.
7. Inability to perform fine and gross movements effectively has the same meaning as in 101.00B2c.
8. Major peripheral joints has the same meaning as in 101.00F.
9. Persistent means that a sign(s) or symptom(s) has continued over time. The precise meaning will depend on the specific immune system disorder, the usual course of the disorder, and the other circumstances of your clinical course.
10. Recurrent means that a condition that previously responded adequately to an appropriate course of treatment returns after a period of remission or regression. The precise meaning, such as the extent of response or remission and the time periods involved, will depend on the specific disease or condition you have, the body system affected, the usual course of the disorder and its treatment, and the other facts of your particular case.
11. Resistant to treatment means that a condition did not respond adequately to an appropriate course of treatment. Whether a response is adequate or a course of treatment is appropriate will depend on the specific disease or condition you have, the body system affected, the usual course of the disorder and its treatment, and the other facts of your particular case.
12. Severe means medical severity as used by the medical community. The term does not have the same meaning as it does when we use it in connection with a finding at the second step of the sequential evaluation process in § 416.924.
D. How do we document and evaluate the listed autoimmune disorders?1. Systemic lupus erythematosus (114.02).
a. General. Systemic lupus erythematosus (SLE) is a chronic inflammatory disease that can affect any organ or body system. It is frequently, but not always, accompanied by constitutional symptoms or signs (severe fatigue, fever, malaise, involuntary weight loss). Major organ or body system involvement can include: Respiratory (pleuritis, pneumonitis), cardiovascular (endocarditis, myocarditis, pericarditis, vasculitis), renal (glomerulonephritis), hematologic (anemia, leukopenia, thrombocytopenia), skin (photosensitivity), neurologic (seizures), mental (anxiety, fluctuating cognition (“lupus fog”), mood disorders, organic brain syndrome, psychosis), or immune system disorders (inflammatory arthritis). Immunologically, there is an array of circulating serum auto-antibodies and pro- and anti-coagulant proteins that may occur in a highly variable pattern.
b. Documentation of SLE. Generally, but not always, the medical evidence will show that your SLE satisfies the criteria in the current “Criteria for the Classification of Systemic Lupus Erythematosus” by the American College of Rheumatology found in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
2. Systemic vasculitis (114.03).
a. General.
(i) Vasculitis is an inflammation of blood vessels. It may occur acutely in association with adverse drug reactions, certain chronic infections, and occasionally, malignancies. More often, it is chronic and the cause is unknown. Symptoms vary depending on which blood vessels are involved. Systemic vasculitis may also be associated with other autoimmune disorders; for example, SLE or dermatomyositis.
(ii) Children can develop the vasculitis of Kawasaki disease, of which the most serious manifestation is formation of coronary artery aneurysms and related complications. We evaluate heart problems related to Kawasaki disease under the criteria in the cardiovascular listings (104.00). Children can also develop the vasculitis of anaphylactoid purpura (Henoch-Schoenlein purpura), which may cause intestinal and renal disorders. We evaluate intestinal and renal disorders related to vasculitis of anaphylactoid purpura under the criteria in the digestive (105.00) or genitourinary (106.00) listings. Other clinical patterns include, but are not limited to, polyarteritis nodosa, Takayasu's arteritis (aortic arch arteritis), and Wegener's granulomatosis.
b. Documentation of systemic vasculitis. Angiography or tissue biopsy confirms a diagnosis of systemic vasculitis when the disease is suspected clinically. When you have had angiography or tissue biopsy for systemic vasculitis, we will make every reasonable effort to obtain reports of the results of that procedure. However, we will not purchase angiography or tissue biopsy.
3. Systemic sclerosis (scleroderma) (114.04).
a. General. Systemic sclerosis (scleroderma) constitutes a spectrum of disease in which thickening of the skin is the clinical hallmark. Raynaud's phenomenon, often medically severe and progressive, is present frequently and may be the peripheral manifestation of a vasospastic abnormality in the heart, lungs, and kidneys. The CREST syndrome (calcinosis, Raynaud's phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) is a variant that may slowly progress over years to the generalized process, systemic sclerosis.
b. Diffuse cutaneous systemic sclerosis. In diffuse cutaneous systemic sclerosis (also known as diffuse scleroderma), major organ or systemic involvement can include the gastrointestinal tract, lungs, heart, kidneys, and muscle in addition to skin or blood vessels. Although arthritis can occur, joint dysfunction results primarily from soft tissue/cutaneous thickening, fibrosis, and contractures.
c. Localized scleroderma (linear scleroderma and morphea).
(i) Localized scleroderma (linear scleroderma and morphea) is more common in children than systemic scleroderma. To assess the severity of the impairment, we need a description of the extent of involvement of linear scleroderma and the location of the lesions. For example, linear scleroderma involving the arm but not crossing any joints is not as functionally limiting as sclerodactyly (scleroderma localized to the fingers). Linear scleroderma of a lower extremity involving skin thickening and atrophy of underlying muscle or bone can result in contractures and leg length discrepancy. In such cases, we may evaluate your impairment under the musculoskeletal listings (101.00).
(ii) When there is isolated morphea of the face causing facial disfigurement from unilateral hypoplasia of the mandible, maxilla, zygoma, or orbit, adjudication may be more appropriate under the criteria in the affected body system, such as special senses and speech (102.00) or mental disorders (112.00).
(iii) Chronic variants of these syndromes include disseminated morphea, Shulman's disease (diffuse fasciitis with eosinophilia), and eosinophilia-myalgia syndrome (often associated with toxins such as toxic oil or contaminated tryptophan), all of which can impose medically severe musculoskeletal dysfunction and may also lead to restrictive pulmonary disease. We evaluate these variants of the disease under the criteria in the musculoskeletal listings (101.00) or respiratory system listings (103.00).
d. Documentation of systemic sclerosis (scleroderma). Documentation involves differentiating the clinical features of systemic sclerosis (scleroderma) from other autoimmune disorders. However, there may be an overlap.
4. Polymyositis and dermatomyositis (114.05).
a. General.
(i) Polymyositis and dermatomyositis are related disorders that are characterized by an inflammatory process in striated muscle, occurring alone or in association with other autoimmune disorders. The most common manifestations are symmetric weakness, and less frequently, pain and tenderness of the proximal limb-girdle (shoulder or pelvic) musculature. There may also be involvement of the cervical, cricopharyngeal, esophageal, intercostal, and diaphragmatic muscles.
(ii) Polymyositis occurs rarely in children; the more common presentation in children is dermatomyositis with symmetric proximal muscle weakness and characteristic skin findings. The clinical course of dermatomyositis can be more severe when it is accompanied by systemic vasculitis rather than just localized to striated muscle. Late in the disease, some children with dermatomyositis develop calcinosis of the skin and subcutaneous tissues, muscles, and joints. We evaluate the involvement of other organs/body systems under the criteria for the listings in the affected body system.
b. Documentation of polymyositis and dermatomyositis. Generally, but not always, polymyositis is associated with elevated serum muscle enzymes (creatine phosphokinase (CPK), aminotransferases, and aldolase), and characteristic abnormalities on electromyography and muscle biopsy. In children, the diagnosis of dermatomyositis is supported largely by medical history, findings on physical examination that include the characteristic skin findings, and elevated serum muscle enzymes. Muscle inflammation or vasculitis depicted on MRI is additional evidence supporting the diagnosis of childhood dermatomyositis. When you have had electromyography, muscle biopsy, or MRI for polymyositis or dermatomyositis, we will make every reasonable effort to obtain reports of the results of that procedure. However, we will not purchase electromyography, muscle biopsy, or MRI.
c. Additional information about how we evaluate polymyositis and dermatomyositis under the listings.
(i) In newborn and younger infants (birth to attainment of age 1), we consider muscle weakness that affects motor skills, such as head control, reaching, grasping, taking solids, or self-feeding, under 114.05A. In older infants and toddlers (age 1 to attainment of age 3), we also consider muscle weakness affecting your ability to roll over, sit, crawl, or walk under 114.05A.
(ii) If you are of preschool age through adolescence (age 3 to attainment of age 18), weakness of your pelvic girdle muscles that results in your inability to rise independently from a squatting or sitting position or to climb stairs may be an indication that you are unable to ambulate effectively. Weakness of your shoulder girdle muscles may result in your inability to perform lifting, carrying, and reaching overhead, and also may seriously affect your ability to perform activities requiring fine movements. We evaluate these limitations under 114.05A.
5. Undifferentiated and mixed connective tissue disease (114.06).
a. General. This listing includes syndromes with clinical and immunologic features of several autoimmune disorders, but which do not satisfy the criteria for any of the specific disorders described. For example, you may have clinical features of SLE and systemic vasculitis, and the serologic (blood test) findings of rheumatoid arthritis. The most common pattern of undifferentiated autoimmune disorders in children is mixed connective tissue disease (MCTD).
b. Documentation of undifferentiated and mixed connective tissue disease. Undifferentiated connective tissue disease is diagnosed when clinical features and serologic (blood test) findings, such as rheumatoid factor or antinuclear antibody (consistent with an autoimmune disorder) are present but do not satisfy the criteria for a specific disease. Children with MCTD have laboratory findings of extremely high antibody titers to extractable nuclear antigen (ENA) or ribonucleoprotein (RNP) without high titers of anti-dsDNA or anti-SM antibodies. There are often clinical findings suggestive of SLE or childhood dermatomyositis. Many children later develop features of scleroderma.
6. Inflammatory arthritis (114.09).
a. General. The spectrum of inflammatory arthritis includes a vast array of disorders that differ in cause, course, and outcome. Clinically, inflammation of major peripheral joints may be the dominant manifestation causing difficulties with ambulation or fine and gross movements; there may be joint pain, swelling, and tenderness. The arthritis may affect other joints, or cause less limitation in ambulation or the performance of fine and gross movements. However, in combination with extra-articular features, including constitutional symptoms or signs (severe fatigue, fever, malaise, involuntary weight loss), inflammatory arthritis may result in an extreme limitation. You may also have impaired growth as a result of the inflammatory arthritis because of its effects on the immature skeleton, open epiphyses, and young cartilage and bone. We evaluate any associated growth impairment under the criteria in 100.00.
b. Inflammatory arthritis involving the axial spine (spondyloarthropathy). In children, inflammatory arthritis involving the axial spine may be associated with disorders such as:
(i) Reactive arthropathies;
(ii) Juvenile ankylosing spondylitis;
(iii) Psoriatic arthritis;
(iv) SEA syndrome (seronegative enthesopathy arthropathy syndrome);
(v) Behçet's disease; and
(vi) Inflammatory bowel disease.
c. Inflammatory arthritis involving the peripheral joints. In children, inflammatory arthritis involving peripheral joints may be associated with disorders such as:
(i) Juvenile rheumatoid arthritis;
(ii) Sjöogren's syndrome;
(iii) Psoriatic arthritis;
(iv) Crystal deposition disorders (gout and pseudogout);
(v) Lyme disease; and
(vi) Inflammatory bowel disease.
d. Documentation of inflammatory arthritis. Generally, but not always, the diagnosis of inflammatory arthritis is based on the clinical features and serologic findings described in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
e. How we evaluate inflammatory arthritis under the listings.
(i) Listing-level severity in 114.09A and 114.09C1 is shown by an impairment that results in an “extreme” (very serious) limitation. In 114.09A, the criterion is satisfied with persistent inflammation or deformity in one major peripheral weight-bearing joint resulting in the inability to ambulate effectively (as defined in 114.00C6) or one major peripheral joint in each upper extremity resulting in the inability to perform fine and gross movements effectively (as defined in 114.00C7). In 114.09C1, if you have the required ankylosis (fixation) of your cervical or dorsolumbar spine, we will find that you have an extreme limitation in your ability to see in front of you, above you, and to the side. Therefore, inability to ambulate effectively is implicit in 114.09C1, even though you might not require bilateral upper limb assistance.
(ii) Listing-level severity is shown in 114.09B and 114.09C2 by inflammatory arthritis that involves various combinations of complications of one or more major peripheral joints or involves other joints, such as inflammation or deformity, extra-articular features, repeated manifestations, and constitutional symptoms and signs. Extra-articular impairments may also meet listings in other body systems.
(iii) Extra-articular features of inflammatory arthritis may involve any body system; for example: Musculoskeletal (heel enthesopathy), ophthalmologic (iridocyclitis, keratoconjunctivitis sicca, uveitis), pulmonary (pleuritis, pulmonary fibrosis or nodules, restrictive lung disease), cardiovascular (aortic valve insufficiency, arrhythmias, coronary arteritis, myocarditis, pericarditis, Raynaud's phenomenon, systemic vasculitis), renal (amyloidosis of the kidney), hematologic (chronic anemia, thrombocytopenia), neurologic (peripheral neuropathy, radiculopathy, spinal cord or cauda equina compression with sensory and motor loss), mental (cognitive dysfunction, poor memory), and immune system (Felty's syndrome (hypersplenism with compromised immune competence)).
(iv) If both inflammation and chronic deformities are present, we evaluate your impairment under the criteria of any appropriate listing.
7. Sjögren's syndrome (114.10).
a. General.
(i) Sjögren's syndrome is an immune-mediated disorder of the exocrine glands. Involvement of the lacrimal and salivary glands is the hallmark feature, resulting in symptoms of dry eyes and dry mouth, and possible complications, such as corneal damage, blepharitis (eyelid inflammation), dysphagia (difficulty in swallowing), dental caries, and the inability to speak for extended periods of time. Involvement of the exocrine glands of the upper airways may result in persistent dry cough.
(ii) Many other organ systems may be involved, including musculoskeletal (arthritis, myositis), respiratory (interstitial fibrosis), gastrointestinal (dysmotility, dysphagia, involuntary weight loss), genitourinary (interstitial cystitis, renal tubular acidosis), skin (purpura, vasculitis,), neurologic (central nervous system disorders, cranial and peripheral neuropathies), mental (cognitive dysfunction, poor memory), and neoplastic (lymphoma). Severe fatigue and malaise are frequently reported. Sjögren's syndrome may be associated with other autoimmune disorders (for example, rheumatoid arthritis or SLE); usually the clinical features of the associated disorder predominate.
b. Documentation of Sjögren's syndrome. If you have Sjögren's syndrome, the medical evidence will generally, but not always, show that your disease satisfies the criteria in the current “Criteria for the Classification of Sjögren's Syndrome” by the American College of Rheumatology found in the most recent edition of the Primer on the Rheumatic Diseases published by the Arthritis Foundation.
E. How do we document and evaluate immune deficiency disorders, excluding HIV infection?1. General.
a. Immune deficiency disorders can be classified as:
(i) Primary (congenital); for example, X-linked agammaglobulinemia, thymic hypoplasia (DiGeorge syndrome), severe combined immunodeficiency (SCID), chronic granulomatous disease (CGD), C1 esterase inhibitor deficiency.
(ii) Acquired; for example, medication-related.
b. Primary immune deficiency disorders are seen mainly in children. However, recent advances in the treatment of these disorders have allowed many affected children to survive well into adulthood. Occasionally, these disorders are first diagnosed in adolescence or adulthood.
2. Documentation of immune deficiency disorders. The medical evidence must include documentation of the specific type of immune deficiency. Documentation may be by laboratory evidence or by other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice.
3. Immune deficiency disorders treated by stem cell transplantation.
a. Evaluation in the first 12 months. If you undergo stem cell transplantation for your immune deficiency disorder, we will consider you disabled until at least 12 months from the date of the transplant.
b. Evaluation after the 12-month period has elapsed. After the 12-month period has elapsed, we will consider any residuals of your immune deficiency disorder as well as any residual impairment(s) resulting from the treatment, such as complications arising from:
(i) Graft-versus-host (GVH) disease.
(ii) Immunosuppressant therapy, such as frequent infections.
(iii) Significant deterioration of other organ systems.
4. Medication-induced immune suppression. Medication effects can result in varying degrees of immune suppression, but most resolve when the medication is ceased. However, if you are prescribed medication for long-term immune suppression, such as after an organ transplant, we will evaluate:
a. The frequency and severity of infections.
b. Residuals from the organ transplant itself, after the 12-month period has elapsed.
c. Significant deterioration of other organ systems.
F. How do we document and evaluate HIV infection? Any child with HIV infection, including one with a diagnosis of acquired immune deficiency syndrome (AIDS), may be found disabled under 114.11 if his or her impairment meets the criteria in that listing or is medically equivalent to the criteria in that listing.
1. Documentation of HIV infection.
a. Definitive documentation of HIV infection. We may document a diagnosis of HIV infection by positive findings on one or more of the following definitive laboratory tests:
(i) HIV antibody screening test (for example, enzyme immunoassay, or EIA), confirmed by a supplemental HIV antibody test such as the Western blot (immunoblot) or immunofluorescence assay, for any child age 18 months or older.
(ii) HIV nucleic acid (DNA or RNA) detection test (for example, polymerase chain reaction, or PCR).
(iii) HIV p24 antigen (p24Ag) test, for any child age 1 month or older.
(iv) Isolation of HIV in viral culture.
(v) Other tests that are highly specific for detection of HIV and that are consistent with the prevailing state of medical knowledge.
b. We will make every reasonable effort to obtain the results of your laboratory testing. Pursuant to § 416.919f of this chapter, we will purchase examinations or tests necessary to make a determination in your claim if no other acceptable documentation exists.
c. Other acceptable documentation of HIV infection. We may also document HIV infection without definitive laboratory evidence.
(i) We will accept a persuasive report from a physician that a positive diagnosis of your HIV infection was confirmed by an appropriate laboratory test(s), such as those described in 114.00F1a. To be persuasive, this report must state that you had the appropriate definitive laboratory test(s) for diagnosing your HIV infection and provide the results. The report must also be consistent with the remaining evidence of record.
(ii) We may also document HIV infection by the medical history, clinical and laboratory findings, and diagnosis(es) indicated in the medical evidence, provided that such documentation is consistent with the prevailing state of medical knowledge and clinical practice and is consistent with the other evidence in your case record. For example, we will accept a diagnosis of HIV infection without definitive laboratory evidence of the HIV infection if you have an opportunistic disease that is predictive of a defect in cell-mediated immunity (for example, toxoplasmosis of the brain or Pneumocystis pneumonia (PCP)), and there is no other known cause of diminished resistance to that disease (for example, long-term steroid treatment or lymphoma). In such cases, we will make every reasonable effort to obtain full details of the history, medical findings, and results of testing.
2. Documentation of the manifestations of HIV infection.
a. Definitive documentation of manifestations of HIV infection. We may document manifestations of HIV infection by positive findings on definitive laboratory tests, such as culture, microscopic examination of biopsied tissue or other material (for example, bronchial washings), serologic tests, or on other generally acceptable definitive tests consistent with the prevailing state of medical knowledge and clinical practice.
b. We will make every reasonable effort to obtain the results of your laboratory testing. Pursuant to § 416.919f of this chapter, we will purchase examinations or tests necessary to make a determination of your claim if no other acceptable documentation exists.
c. Other acceptable documentation of manifestations of HIV infection. We may also document manifestations of HIV infection without definitive laboratory evidence.
(i) We will accept a persuasive report from a physician that a positive diagnosis of your manifestation of HIV infection was confirmed by an appropriate laboratory test(s). To be persuasive, this report must state that you had the appropriate definitive laboratory test(s) for diagnosing your manifestation of HIV infection and provide the results. The report must also be consistent with the remaining evidence of record.
(ii) We may also document manifestations of HIV infection without the definitive laboratory evidence described in 114.00F2a, provided that such documentation is consistent with the prevailing state of medical knowledge and clinical practice and is consistent with the other evidence in your case record. For example, many conditions are now commonly diagnosed based on some or all of the following: Medical history, clinical manifestations, laboratory findings (including appropriate medically acceptable imaging), and treatment responses. In such cases, we will make every reasonable effort to obtain full details of the history, medical findings, and results of testing.
3. Disorders associated with HIV infection (114.11A-E).
a. Multicentric Castleman disease (MCD, 114.11A) affects multiple groups of lymph nodes and organs containing lymphoid tissue. This widespread involvement distinguishes MCD from localized (or unicentric) Castleman disease, which affects only a single set of lymph nodes. While not a cancer, MCD is known as a lymphoproliferative disorder. Its clinical presentation and progression is similar to that of lymphoma, and its treatment may include radiation or chemotherapy. We require characteristic findings on microscopic examination of the biopsied lymph nodes or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis. Localized (or unicentric) Castleman disease does not meet or medically equal the criterion in 114.11A, but we may evaluate it under the criteria in 114.11G or 14.11I in part A.
b. Primary central nervous system lymphoma (PCNSL, 114.11B) originates in the brain, spinal cord, meninges, or eye. Imaging tests (for example, MRI) of the brain, while not diagnostic, may show a single lesion or multiple lesions in the white matter of the brain. We require characteristic findings on microscopic examination of the cerebral spinal fluid or of the biopsied brain tissue, or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
c. Primary effusion lymphoma (PEL, 114.11C) is also known as body cavity lymphoma. We require characteristic findings on microscopic examination of the effusion fluid or of the biopsied tissue from the affected internal organ, or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
d. Progressive multifocal leukoencephalopathy (PML, 114.11D) is a progressive neurological degenerative syndrome caused by the John Cunningham (JC) virus in immunosuppressed children. Clinical findings of PML include clumsiness, progressive weakness, and visual and speech changes. Personality and cognitive changes may also occur. We require appropriate clinical findings, characteristic white matter lesions on MRI, and a positive PCR test for the JC virus in the cerebrospinal fluid to establish the diagnosis. We also accept a positive brain biopsy for JC virus or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
e. Pulmonary Kaposi sarcoma (Kaposi sarcoma in the lung, 114.11E) is the most serious form of Kaposi sarcoma (KS). Other internal KS tumors (for example, tumors of the gastrointestinal tract) have a more variable prognosis. We require characteristic findings on microscopic examination of the induced sputum, bronchoalveolar lavage washings, or of the biopsied transbronchial tissue, or other generally acceptable methods consistent with the prevailing state of medical knowledge and clinical practice to establish the diagnosis.
4. CD4 measurement (114.11F). To evaluate your HIV infection under 114.11F, we require one measurement of your absolute CD4 count (also known as CD4 count or CD4+ T-helper lymphocyte count) or CD4 percentage for children from birth to attainment of age 5, or one measurement of your absolute CD4 count for children from age 5 to attainment of age 18. These measurements (absolute CD4 count or CD4 percentage) must occur within the period we are considering in connection with your application or continuing disability review. If you have more than one CD4 measurement within this period, we will use your lowest absolute CD4 count or your lowest CD4 percentage.
5. Complications of HIV infection requiring hospitalization (114.11G).
a. Complications of HIV infection may include infections (common or opportunistic), cancers, and other conditions. Examples of complications that may result in hospitalization include: Depression; diarrhea; immune reconstitution inflammatory syndrome; malnutrition; and PCP and other severe infections.
b. Under 114.11G, we require three hospitalizations within a 12-month period that are at least 30 days apart and that result from a complication(s) of HIV infection. The hospitalizations may be for the same complication or different complications of HIV infection and are not limited to the examples of complications that may result in hospitalization listed in 114.00F5a. All three hospitalizations must occur within the period we are considering in connection with your application or continuing disability review. Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
c. We will use the rules on medical equivalence in § 416.926 of this chapter to evaluate your HIV infection if you have fewer, but longer, hospitalizations, or more frequent, but shorter, hospitalizations, or if you receive nursing, rehabilitation, or other care in alternative settings.
6. Neurological manifestations specific to children (114.11H). The methods of identifying and evaluating neurological manifestations may vary depending on a child's age. For example, in an infant, impaired brain growth can be documented by a decrease in the growth rate of the head. In an older child, impaired brain growth may be documented by brain atrophy on a CT scan or MRI. Neurological manifestations may present in the loss of acquired developmental milestones (developmental regression) in infants and young children or, in the loss of acquired intellectual abilities in school-age children and adolescents. A child may demonstrate loss of intellectual abilities by a decrease in IQ scores, by forgetting information previously learned, by inability to learn new information, or by a sudden onset of a new learning disability. When infants and young children present with serious developmental delays (without regression), we evaluate the child's impairment(s) under 112.00.
7. Growth failure due to HIV immune suppression (114.11I).
a. To evaluate growth failure due to HIV immune suppression, we require documentation of the laboratory values described in 114.11I1 and the growth measurements in 114.11I2 or 114.11I3 within the same consecutive 12-month period. The dates of laboratory findings may be different from the dates of growth measurements.
b. Under 114.11I2 and 114.11I3, we use the appropriate table under 105.08B in the digestive system to determine whether a child's growth is less than the third percentile.
(i) For children from birth to attainment of age 2, we use the weight-for-length table corresponding to the child's gender (Table I or Table II).
(ii) For children from age 2 to attainment of age 18, we use the body mass index (BMI)-for-age corresponding to the child's gender (Table III or Table IV).
(iii) BMI is the ratio of a child's weight to the square of his or her height. We calculate BMI using the formulas in 105.00G2c.
G. How do we consider the effects of treatment in evaluating your autoimmune disorder, immune deficiency disorder, or HIV infection?1. General. If your impairment does not otherwise meet the requirements of a listing, we will consider your medical treatment in terms of its effectiveness in improving the signs, symptoms, and laboratory abnormalities of your specific immune system disorder or its manifestations, and in terms of any side effects that limit your functioning. We will make every reasonable effort to obtain a specific description of the treatment you receive (including surgery) for your immune system disorder. We consider:
a. The effects of medications you take.
b. Adverse side effects (acute and chronic).
c. The intrusiveness and complexity of your treatment (for example, the dosing schedule, need for injections).
d. The effect of treatment on your mental functioning (for example, cognitive changes, mood disturbance).
e. Variability of your response to treatment (see 114.00G2).
f. The interactive and cumulative effects of your treatments. For example, many children with immune system disorders receive treatment both for their immune system disorders and for the manifestations of the disorders or co-occurring impairments, such as treatment for HIV infection and hepatitis C. The interactive and cumulative effects of these treatments may be greater than the effects of each treatment considered separately.
g. The duration of your treatment.
h. Any other aspects of treatment that may interfere with your ability to function.
2. Variability of your response to treatment. Your response to treatment and the adverse or beneficial consequences of your treatment may vary widely. The effects of your treatment may be temporary or long term. For example, some children may show an initial positive response to a drug or combination of drugs followed by a decrease in effectiveness. When we evaluate your response to treatment and how your treatment may affect you, we consider such factors as disease activity before treatment, requirements for changes in therapeutic regimens, the time required for therapeutic effectiveness of a particular drug or drugs, the limited number of drug combinations that may be available for your impairment(s), and the time-limited efficacy of some drugs. For example, a child with HIV infection or another immune deficiency disorder who develops otitis media may not respond to the same antibiotic regimen used in treating children without HIV infection or another immune deficiency disorder, or may not respond to an antibiotic that he or she responded to before. Therefore, we must consider the effects of your treatment on an individual basis, including the effects of your treatment on your ability to function.
3. How we evaluate the effects of treatment for autoimmune disorders on your ability to function. Some medications may have acute or long-term side effects. When we consider the effects of corticosteroids or other treatments for autoimmune disorders on your ability to function, we consider the factors in 114.00G1 and 114.00G2. Long-term corticosteroid treatment can cause ischemic necrosis of bone, posterior subcapsular cataract, impaired growth, weight gain, glucose intolerance, increased susceptibility to infection, and osteopenia that may result in a loss of function. In addition, medications used in the treatment of autoimmune disorders may also have effects on mental functioning, including cognition (for example, memory), concentration, and mood.
4. How we evaluate the effects of treatment for immune deficiency disorders, excluding HIV infection, on your ability to function. When we consider the effects of your treatment for your immune deficiency disorder on your ability to function, we consider the factors in 114.00G1 and 114.00G2. A frequent need for treatment such as intravenous immunoglobulin and gamma interferon therapy can be intrusive and interfere with your ability to function. We will also consider whether you have chronic side effects from these or other medications, including severe fatigue, fever, headaches, high blood pressure, joint swelling, muscle aches, nausea, shortness of breath, or limitations in mental function including cognition (for example, memory) concentration, and mood.
5. How we evaluate the effects of treatment for HIV infection on your ability to function.
a. General. When we consider the effects of antiretroviral drugs (including the effects of highly active antiretroviral therapy (HAART)) and the effects of treatments for the manifestations of HIV infection on your ability to function, we consider the factors in 114.00G1 and 114.00G2. Side effects of antiretroviral drugs include, but are not limited to: Bone marrow suppression, pancreatitis, gastrointestinal intolerance (nausea, vomiting, diarrhea), neuropathy, rash, hepatotoxicity, lipodystrophy (fat redistribution, such as “buffalo hump”), glucose intolerance, and lactic acidosis. In addition, medications used in the treatment of HIV infection may also have effects on mental functioning, including cognition (for example, memory), concentration, and mood, and may result in malaise, severe fatigue, joint and muscle pain, and insomnia. The symptoms of HIV infection and the side effects of medication may be indistinguishable from each other. We will consider all of your functional limitations, whether they result from your symptoms or signs of HIV infection or the side effects of your treatment.
b. Structured treatment interruptions. A structured treatment interruption (STI, also called a “drug holiday”) is a treatment practice during which your treating source advises you to stop taking your medications temporarily. An STI in itself does not imply that your medical condition has improved; nor does it imply that you are noncompliant with your treatment because you are following your treating source's advice. Therefore, if you have stopped taking medication because your treating source prescribed or recommended an STI, we will not find that you are failing to follow treatment or draw inferences about the severity of your impairment on this fact alone. We will consider why your treating source has prescribed or recommended an STI and all the other information in your case record when we determine the severity of your impairment.
6. When there is no record of ongoing treatment. If you have not received ongoing treatment or have not had an ongoing relationship with the medical community despite the existence of a severe impairment(s), we will evaluate the medical severity and duration of your immune system disorder on the basis of the current objective medical evidence and other evidence in your case record, taking into consideration your medical history, symptoms, clinical and laboratory findings, and medical source opinions. If you have just begun treatment and we cannot determine whether you are disabled based on the evidence we have, we may need to wait to determine the effect of the treatment on your ability to develop and function in an age-appropriate manner. The amount of time we need to wait will depend on the facts of your case. If you have not received treatment, you may not be able to show an impairment that meets the criteria of one of the immune system disorders listings, but your immune system disorder may medically equal a listing or functionally equal the listings.
H. How do we consider your symptoms, including your pain, severe fatigue, and malaise?Your symptoms, including pain, severe fatigue, and malaise, may be important factors in our determination whether your immune system disorder(s) meets or medically equals a listing or in our determination whether you otherwise have marked and severe functional limitations. In order for us to consider your symptoms, you must have medical signs or laboratory findings showing the existence of a medically determinable impairment(s) that could reasonably be expected to produce the symptoms. If you have such an impairment(s), we will evaluate the intensity, persistence, and functional effects of your symptoms using the rules throughout 114.00 and in our other regulations. See §§ 416.921 and 416.929. Additionally, when we assess the credibility of your complaints about your symptoms and their functional effects, we will not draw any inferences from the fact that you do not receive treatment or that you are not following treatment without considering all of the relevant evidence in your case record, including any explanations you provide that may explain why you are not receiving or following treatment.
I. How do we consider the impact of your immune system disorder on your functioning?1. We will consider all relevant information in your case record to determine the full impact of your immune system disorder, including HIV infection, on your ability to function. Functional limitation may result from the impact of the disease process itself on your mental functioning, physical functioning, or both your mental and physical functioning. This could result from persistent or intermittent symptoms, such as depression, diarrhea, severe fatigue, or pain, resulting in a limitation of your ability to acquire information, to concentrate, to persevere at a task, to interact with others, to move about, or to cope with stress. You may also have limitations because of your treatment and its side effects (see 114.00G).
2. Important factors we will consider when we evaluate your functioning include, but are not limited to: Your symptoms (see 114.00H), the frequency and duration of manifestations of your immune system disorder, periods of exacerbation and remission, and the functional impact of your treatment, including the side effects of your medication (see 114.00G). See §§ 416.924a and 416.926a of this chapter for additional guidance on the factors we consider when we evaluate your functioning.
3. We will use the rules in §§ 416.924a and 416.926a of this chapter to evaluate your functional limitations and determine whether your impairment functionally equals the listings.
J. How do we evaluate your immune system disorder when it does not meet one of these listings?1. These listings are only examples of immune system disorders that we consider severe enough to result in marked and severe functional limitations. If your impairment(s) does not meet the criteria of any of these listings, we must also consider whether you have an impairment(s) that satisfies the criteria of a listing in another body system.
2. Children with immune system disorders, including HIV infection, may manifest signs or symptoms of a mental impairment or of another physical impairment. For example, HIV infection may accelerate the onset of conditions such as diabetes or affect the course of or treatment options for diseases such as cardiovascular disease or hepatitis. We may evaluate these impairments under the affected body system. For example, we will evaluate:
a. Growth impairment under 100.00.
b. Musculoskeletal involvement, such as surgical reconstruction of a joint, under 101.00.
c. Ocular involvement, such as dry eye, under 102.00.
d. Respiratory impairments, such as pleuritis, under 103.00.
e. Cardiovascular impairments, such as cardiomyopathy, under 104.00.
f. Digestive impairments, such as hepatitis (including hepatitis C) or weight loss as a result of HIV infection that affects the digestive system, under 105.00.
g. Genitourinary impairments, such as nephropathy, under 106.00.
h. Hematologic abnormalities, such as anemia, granulocytopenia, and thrombocytopenia, under 107.00.
i. Skin impairments, such as persistent fungal and other infectious skin eruptions, and photosensitivity, under 108.00.
j. Neurologic impairments, such as neuropathy or seizures, under 111.00.
k. Mental disorders, such as depression, anxiety, or cognitive deficits, under 112.00.
l. Allergic disorders, such as asthma or atopic dermatitis, under 103.00 or 108.00 or under the criteria in another affected body system.
m. Syphilis or neurosyphilis under the criteria for the affected body system, for example, 102.00 Special senses and speech, 104.00 Cardiovascular system, or 111.00 Neurological.
3. If you have a severe medically determinable impairment(s) that does not meet a listing, we will determine whether your impairment(s) medically equals a listing. (See § 416.926.) If it does not, we will also consider whether you have an impairment(s) that functionally equals the listings. (See § 416.926a.) We use the rules in § 416.994a when we decide whether you continue to be disabled.
114.01 Category of Impairments, Immune System Disorders.
114.02 Systemic lupus erythematosus, as described in 114.00D1. With involvement of two or more organs/body systems, and with:
A. One of the organs/body systems involved to at least a moderate level of severity;
ANDB. At least two of the constitutional symptoms and signs (severe fatigue, fever, malaise, or involuntary weight loss).
114.03 Systemic vasculitis, as described in 114.00D2. With involvement of two or more organs/body systems, and with:
A. One of the organs/body systems involved to at least a moderate level of severity;
ANDB. At least two of the constitutional symptoms and signs (severe fatigue, fever, malaise, or involuntary weight loss).
114.04 Systemic sclerosis (scleroderma). As described in 114.00D3. With:
A. Involvement of two or more organs/body systems, with:
1. One of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orB. With one of the following:
1. Toe contractures or fixed deformity of one or both feet, resulting in the inability to ambulate effectively as defined in 114.00C6; or
2. Finger contractures or fixed deformity in both hands, resulting in the inability to perform fine and gross movements effectively as defined in 114.00C7; or
3. Atrophy with irreversible damage in one or both lower extremities, resulting in the inability to ambulate effectively as defined in 114.00C6; or
4. Atrophy with irreversible damage in both upper extremities, resulting in the inability to perform fine and gross movements effectively as defined in 114.00C7.
orC. Raynaud's phenomenon, characterized by:
1. Gangrene involving at least two extremities; or
2. Ischemia with ulcerations of toes or fingers, resulting in the inability to ambulate effectively or to perform fine and gross movements effectively as defined in 114.00C6 and 114.00C7.
114.05 Polymyositis and dermatomyositis. As described in 114.00D4. With:
A. Proximal limb-girdle (pelvic or shoulder) muscle weakness, resulting in inability to ambulate effectively or inability to perform fine and gross movements effectively as defined in 114.00C6 and 114.00C7.
orB. Impaired swallowing (dysphagia) with aspiration due to muscle weakness.
orC. Impaired respiration due to intercostal and diaphragmatic muscle weakness.
orD. Diffuse calcinosis with limitation of joint mobility or intestinal motility.
114.06 Undifferentiated and mixed connective tissue disease, as described in 114.00D5. With involvement of two or more organs/body systems, and with:
A. One of the organs/body systems involved to at least a moderate level of severity;
ANDB. At least two of the constitutional symptoms and signs (severe fatigue, fever, malaise, or involuntary weight loss).
114.07 Immune deficiency disorders, excluding HIV infection. As described in 114.00E. With:
A. One or more of the following infections. The infection(s) must either be resistant to treatment or require hospitalization or intravenous treatment three or more times in a 12-month period.
1. Sepsis; or
2. Meningitis; or
3. Pneumonia; or
4. Septic arthritis; or
5. Endocarditis; or
6. Sinusitis documented by appropriate medically acceptable imaging.
orB. Stem cell transplantation as described under 114.00E3. Consider under a disability until at least 12 months from the date of transplantation. Thereafter, evaluate any residual impairment(s) under the criteria for the affected body system.
114.08 [Reserved]
114.09 Inflammatory arthritis. As described in 114.00D6. With:
A. Persistent inflammation or persistent deformity of:
1. One or more major peripheral weight-bearing joints resulting in the inability to ambulate effectively (as defined in 114.00C6); or
2. One or more major peripheral joints in each upper extremity resulting in the inability to perform fine and gross movements effectively (as defined in 114.00C7).
orB. Inflammation or deformity in one or more major peripheral joints with:
1. Involvement of two or more organs/body systems with one of the organs/body systems involved to at least a moderate level of severity; and
2. At least two of the constitutional symptoms or signs (severe fatigue, fever, malaise, or involuntary weight loss).
orC. Ankylosing spondylitis or other spondyloarthropathies, with:
1. Ankylosis (fixation) of the dorsolumbar or cervical spine as shown by appropriate medically acceptable imaging and measured on physical examination at 45° or more of flexion from the vertical position (zero degrees); or
2. Ankylosis (fixation) of the dorsolumbar or cervical spine as shown by appropriate medically acceptable imaging and measured on physical examination at 30° or more of flexion (but less than 45°) measured from the vertical position (zero degrees), and involvement of two or more organs/body systems with one of the organs/body systems involved to at least a moderate level of severity.
114.10 Sjögren's syndrome, as described in 114.00D7. With involvement of two or more organs/body systems, and with:
A. One of the organs/body systems involved to at least a moderate level of severity;
ANDB. At least two of the constitutional symptoms and signs (severe fatigue, fever, malaise, or involuntary weight loss).
114.11 Human immunodeficiency virus (HIV) infection. With documentation as described in 114.00F1 and one of the following:
A. Multicentric (not localized or unicentric) Castleman disease affecting multiple groups of lymph nodes or organs containing lymphoid tissue (see 114.00F3a).
ORB. Primary central nervous system lymphoma (see 114.00F3b).
ORC. Primary effusion lymphoma (see 114.00F3c).
ORD. Progressive multifocal leukoencephalopathy (see 114.00F3d).
ORE. Pulmonary Kaposi sarcoma (see 114.00F3e).
ORF. Absolute CD4 count or CD4 percentage (see 114.00F4):
1. For children from birth to attainment of age 1, absolute CD4 count of 500 cells/mm 3 or less, or CD4 percentage of less than 15 percent; or
2. For children from age 1 to attainment of age 5, absolute CD4 count of 200 cells/mm 3 or less, or CD4 percentage of less than 15 percent; or
3. For children from age 5 to attainment of age 18, absolute CD4 count of 50 cells/mm 3 or less.
ORG. Complication(s) of HIV infection requiring at least three hospitalizations within a 12-month period and at least 30 days apart (see 114.00F5). Each hospitalization must last at least 48 hours, including hours in a hospital emergency department immediately before the hospitalization.
ORH. A neurological manifestation of HIV infection (for example, HIV encephalopathy or peripheral neuropathy) (see 114.00F6) resulting in one of the following:
1. Loss of previously acquired developmental milestones or intellectual ability (including the sudden onset of a new learning disability), documented on two examinations at least 60 days apart; or
2. Progressive motor dysfunction affecting gait and station or fine and gross motor skills, documented on two examinations at least 60 days apart; or
3. Microcephaly with head circumference that is less than the third percentile for age, documented on two examinations at least 60 days apart; or
4. Brain atrophy, documented by appropriate medically acceptable imaging.
ORI. Immune suppression and growth failure (see 114.00F7) documented by 1 and 2, or by 1 and 3:
1. CD4 measurement:
a. For children from birth to attainment of age 5, CD4 percentage of less than 20 percent; or
b. For children from age 5 to attainment of age 18, absolute CD4 count of less than 200 cells/mm 3 or CD4 percentage of less than 14 percent; and
2. For children from birth to attainment of age 2, three weight-for-length measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate weight-for-length table under 105.08B1; or
3. For children from age 2 to attainment of age 18, three BMI-for-age measurements that are:
a. Within a consecutive 12-month period; and
b. At least 60 days apart; and
c. Less than the third percentile on the appropriate BMI-for-age table under 105.08B2.
[50 FR 35066, Aug. 28, 1985] Editorial Note:For Federal Register citations affecting appendix 1, see the List of CFR Sections Affected, which appears in the Finding Aids section of the printed volume and at www.govinfo.gov.Appendix 2 to Subpart P of Part 404 - Medical-Vocational Guidelines
20:2.0.1.1.5.16.200.105.12 :
Appendix 2 to Subpart P of Part 404 - Medical-Vocational Guidelines Sec. 200.00 Introduction. 201.00 Maximum sustained work capability limited to sedentary work as a result of severe medically determinable impairment(s). 202.00 Maximum sustained work capability limited to light work as a result of severe medically determinable impairment(s). 203.00 Maximum sustained work capability limited to medium work as a result of severe medically determinable impairment(s). 204.00 Maximum sustained work capability limited to heavy work (or very heavy work) as a result of severe medically determinable impairment(s).200.00 Introduction. (a) The following rules reflect the major functional and vocational patterns which are encountered in cases which cannot be evaluated on medical considerations alone, where an individual with a severe medically determinable physical or mental impairment(s) is not engaging in substantial gainful activity and the individual's impairment(s) prevents the performance of his or her vocationally relevant past work. They also reflect the analysis of the various vocational factors (i.e., age, education, and work experience) in combination with the individual's residual functional capacity (used to determine his or her maximum sustained work capability for sedentary, light, medium, heavy, or very heavy work) in evaluating the individual's ability to engage in substantial gainful activity in other than his or her vocationally relevant past work. Where the findings of fact made with respect to a particular individual's vocational factors and residual functional capacity coincide with all of the criteria of a particular rule, the rule directs a conclusion as to whether the individual is or is not disabled. However, each of these findings of fact is subject to rebuttal and the individual may present evidence to refute such findings. Where any one of the findings of fact does not coincide with the corresponding criterion of a rule, the rule does not apply in that particular case and, accordingly, does not direct a conclusion of disabled or not disabled. In any instance where a rule does not apply, full consideration must be given to all of the relevant facts of the case in accordance with the definitions and discussions of each factor in the appropriate sections of the regulations.
(b) The existence of jobs in the national economy is reflected in the “Decisions” shown in the rules; i.e., in promulgating the rules, administrative notice has been taken of the numbers of unskilled jobs that exist throughout the national economy at the various functional levels (sedentary, light, medium, heavy, and very heavy) as supported by the “Dictionary of Occupational Titles” and the “Occupational Outlook Handbook,” published by the Department of Labor; the “County Business Patterns” and “Census Surveys” published by the Bureau of the Census; and occupational surveys of light and sedentary jobs prepared for the Social Security Administration by various State employment agencies. Thus, when all factors coincide with the criteria of a rule, the existence of such jobs is established. However, the existence of such jobs for individuals whose remaining functional capacity or other factors do not coincide with the criteria of a rule must be further considered in terms of what kinds of jobs or types of work may be either additionally indicated or precluded.
(c) In the application of the rules, the individual's residual functional capacity (i.e., the maximum degree to which the individual retains the capacity for sustained performance of the physical-mental requirements of jobs), age, education, and work experience must first be determined. When assessing the person's residual functional capacity, we consider his or her symptoms (such as pain), signs, and laboratory findings together with other evidence we obtain.
(d) The correct disability decision (i.e., on the issue of ability to engage in substantial gainful activity) is found by then locating the individual's specific vocational profile. If an individual's specific profile is not listed within this appendix 2, a conclusion of disabled or not disabled is not directed. Thus, for example, an individual's ability to engage in substantial gainful work where his or her residual functional capacity falls between the ranges of work indicated in the rules (e.g., the individual who can perform more than light but less than medium work), is decided on the basis of the principles and definitions in the regulations, giving consideration to the rules for specific case situations in this appendix 2. These rules represent various combinations of exertional capabilities, age, education and work experience and also provide an overall structure for evaluation of those cases in which the judgments as to each factor do not coincide with those of any specific rule. Thus, when the necessary judgments have been made as to each factor and it is found that no specific rule applies, the rules still provide guidance for decisionmaking, such as in cases involving combinations of impairments. For example, if strength limitations resulting from an individual's impairment(s) considered with the judgments made as to the individual's age, education and work experience correspond to (or closely approximate) the factors of a particular rule, the adjudicator then has a frame of reference for considering the jobs or types of work precluded by other, nonexertional impairments in terms of numbers of jobs remaining for a particular individual.
(e) Since the rules are predicated on an individual's having an impairment which manifests itself by limitations in meeting the strength requirements of jobs, they may not be fully applicable where the nature of an individual's impairment does not result in such limitations, e.g., certain mental, sensory, or skin impairments. In addition, some impairments may result solely in postural and manipulative limitations or environmental restrictions. Environmental restrictions are those restrictions which result in inability to tolerate some physical feature(s) of work settings that occur in certain industries or types of work, e.g., an inability to tolerate dust or fumes.
(1) In the evaluation of disability where the individual has solely a nonexertional type of impairment, determination as to whether disability exists shall be based on the principles in the appropriate sections of the regulations, giving consideration to the rules for specific case situations in this appendix 2. The rules do not direct factual conclusions of disabled or not disabled for individuals with solely nonexertional types of impairments.
(2) However, where an individual has an impairment or combination of impairments resulting in both strength limitations and nonexertional limitations, the rules in this subpart are considered in determining first whether a finding of disabled may be possible based on the strength limitations alone and, if not, the rule(s) reflecting the individual's maximum residual strength capabilities, age, education, and work experience provide a framework for consideration of how much the individual's work capability is further diminished in terms of any types of jobs that would be contraindicated by the nonexertional limitations. Also, in these combinations of nonexertional and exertional limitations which cannot be wholly determined under the rules in this appendix 2, full consideration must be given to all of the relevant facts in the case in accordance with the definitions and discussions of each factor in the appropriate sections of the regulations, which will provide insight into the adjudicative weight to be accorded each factor.
201.00 Maximum sustained work capability limited to sedentary work as a result of severe medically determinable impairment(s). (a) Most sedentary occupations fall within the skilled, semi-skilled, professional, administrative, technical, clerical, and benchwork classifications. Approximately 200 separate unskilled sedentary occupations can be identified, each representing numerous jobs in the national economy. Approximately 85 percent of these jobs are in the machine trades and benchwork occupational categories. These jobs (unskilled sedentary occupations) may be performed after a short demonstration or within 30 days.
(b) These unskilled sedentary occupations are standard within the industries in which they exist. While sedentary work represents a significantly restricted range of work, this range in itself is not so prohibitively restricted as to negate work capability for substantial gainful activity.
(c) Vocational adjustment to sedentary work may be expected where the individual has special skills or experience relevant to sedentary work or where age and basic educational competences provide sufficient occupational mobility to adapt to the major segment of unskilled sedentary work. Inability to engage in substantial gainful activity would be indicated where an individual who is restricted to sedentary work because of a severe medically determinable impairment lacks special skills or experience relevant to sedentary work, lacks educational qualifications relevant to most sedentary work (e.g., has a limited education or less) and the individual's age, though not necessarily advanced, is a factor which significantly limits vocational adaptability.
(d) The adversity of functional restrictions to sedentary work at advanced age (55 and over) for individuals with no relevant past work or who can no longer perform vocationally relevant past work and have no transferable skills, warrants a finding of disabled in the absence of the rare situation where the individual has recently completed education which provides a basis for direct entry into skilled sedentary work. Advanced age and a history of unskilled work or no work experience would ordinarily offset any vocational advantages that might accrue by reason of any remote past education, whether it is more or less than limited education.
(e) The presence of acquired skills that are readily transferable to a significant range of skilled work within an individual's residual functional capacity would ordinarily warrant a finding of ability to engage in substantial gainful activity regardless of the adversity of age, or whether the individual's formal education is commensurate with his or her demonstrated skill level. The acquisition of work skills demonstrates the ability to perform work at the level of complexity demonstrated by the skill level attained regardless of the individual's formal educational attainments.
(f) In order to find transferability of skills to skilled sedentary work for individuals who are of advanced age (55 and over), there must be very little, if any, vocational adjustment required in terms of tools, work processes, work settings, or the industry.
(g) Individuals approaching advanced age (age 50-54) may be significantly limited in vocational adaptability if they are restricted to sedentary work. When such individuals have no past work experience or can no longer perform vocationally relevant past work and have no transferable skills, a finding of disabled ordinarily obtains. However, recently completed education which provides for direct entry into sedentary work will preclude such a finding. For this age group, even a high school education or more (ordinarily completed in the remote past) would have little impact for effecting a vocational adjustment unless relevant work experience reflects use of such education.
(h)(1) The term younger individual is used to denote an individual age 18 through 49. For individuals who are age 45-49, age is a less advantageous factor for making an adjustment to other work than for those who are age 18-44. Accordingly, a finding of “disabled” is warranted for individuals age 45-49 who:
(i) Are restricted to sedentary work,
(ii) Are unskilled or have no transferable skills,
(iii) Have no past relevant work or can no longer perform past relevant work, and
(iv) Are illiterate.
(2) For individuals who are under age 45, age is a more advantageous factor for making an adjustment to other work. It is usually not a significant factor in limiting such individual's ability to make an adjustment to other work, including an adjustment to unskilled sedentary work, even when the individuals are illiterate.
(3) Nevertheless, a decision of “disabled” may be appropriate for some individuals under age 45 (or individuals age 45-49 for whom rule 201.17 does not direct a decision of disabled) who do not have the ability to perform a full range of sedentary work. However, the inability to perform a full range of sedentary work does not necessarily equate with a finding of “disabled.” Whether an individual will be able to make an adjustment to other work requires an adjudicative assessment of factors such as the type and extent of the individual's limitations or restrictions and the extent of the erosion of the occupational base. It requires an individualized determination that considers the impact of the limitations or restrictions on the number of sedentary, unskilled occupations or the total number of jobs to which the individual may be able to adjust, considering his or her age, education and work experience, including any transferable skills or education providing for direct entry into skilled work.
(4) “Sedentary work” represents a significantly restricted range of work, and individuals with a maximum sustained work capability limited to sedentary work have very serious functional limitations. Therefore, as with any case, a finding that an individual is limited to less than the full range of sedentary work will be based on careful consideration of the evidence of the individual's medical impairment(s) and the limitations and restrictions attributable to it. Such evidence must support the finding that the individual's residual functional capacity is limited to less than the full range of sedentary work.
(i) While illiteracy may significantly limit an individual's vocational scope, the primary work functions in most unskilled occupations involve working with things (rather than with data or people). In these work functions, education has the least significance. Similarly the lack of relevant work experience would have little significance since the bulk of unskilled jobs require no qualifying work experience. Thus, the functional capacity for a full range of sedentary work represents sufficient numbers of jobs to indicate substantial vocational scope for those individuals age 18-44, even if they are illiterate.
Table No. 1 - Residual Functional Capacity: Maximum Sustained Work Capability Limited to Sedentary Work as a Result of Severe Medically Determinable Impairment(s)
| Rule | Age | Education | Previous work experience | Decision |
|---|---|---|---|---|
| 201.01 | Advanced age | Limited or less | Unskilled or none | Disabled |
| 201.02 | ......do | ......do | Skilled or semiskilled - skills not transferable 1 | Do. |
| 201.03 | ......do | ......do | Skilled or semiskilled - skills transferable 1 | Not disabled |
| 201.04 | ......do | High school graduate or more - does not provide for direct entry into skilled work 2 | Unskilled or none | Disabled |
| 201.05 | ......do | High school graduate or more - provides for direct entry into skilled work 2 | ......do | Not disabled |
| 201.06 | ......do | High school graduate or more - does not provide for direct entry into skilled work 2 | Skilled or semiskilled - skills not transferable 1 | Disabled |
| 201.07 | ......do | ......do | Skilled or semiskilled - skills transferable 1 | Not disabled |
| 201.08 | ......do | High school graduate or more - provides for direct entry into skilled work 2 | Skilled or semiskilled - skills not transferable 1 | Do. |
| 201.09 | Closely approaching advanced age | Limited or less | Unskilled or none | Disabled |
| 201.10 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 201.11 | ......do | ......do | Skilled or semiskilled - skills transferable | Not disabled |
| 201.12 | ......do | High school graduate or more - does not provide for direct entry into skilled work 3 | Unskilled or none | Disabled |
| 201.13 | ......do | High school graduate or more - provides for direct entry into skilled work 3 | ......do | Not disabled |
| 201.14 | ......do | High school graduate or more - does not provide for direct entry into skilled work 3 | Skilled or semiskilled - skills not transferable | Disabled |
| 201.15 | ......do | ......do | Skilled or semiskilled - skills transferable | Not disabled |
| 201.16 | ......do | High school graduate or more - provides for direct entry into skilled work 3 | Skilled or semiskilled - skills not transferable | Do. |
| 201.17 | Younger individual age 45-49 | Illiterate | Unskilled or none | Disabled. |
| 201.18 | ......do | Limited or Marginal, but not Illiterate | ......do | Not disabled. |
| 201.19 | ......do | Limited or less | Skilled or semiskilled - skills not transferable | Do. |
| 201.20 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 201.21 | ......do | High school graduate or more | Skilled or semiskilled - skills not transferable | Do. |
| 201.22 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 201.23 | Younger individual age 18-44 | Illiterate | Unskilled or none | 4 Do. |
| 201.24 | ......do | Limited or Marginal, but not Illiterate | ......do | 4 Do. |
| 201.25 | ......do | Limited or less | Skilled or semiskilled - skills not transferable | Do. 4 |
| 201.26 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. 4 |
| 201.27 | ......do | High school graduate or more | Unskilled or none | Do. 4 |
| 201.28 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. 4 |
| 201.29 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. 4 |
1 See 201.00(f).
2 See 201.00(d).
3 See 201.00(g).
4 See 201.00(h).
202.00 Maximum sustained work capability limited to light work as a result of severe medically determinable impairment(s). (a) The functional capacity to perform a full range of light work includes the functional capacity to perform sedentary as well as light work. Approximately 1,600 separate sedentary and light unskilled occupations can be identified in eight broad occupational categories, each occupation representing numerous jobs in the national economy. These jobs can be performed after a short demonstration or within 30 days, and do not require special skills or experience.
(b) The functional capacity to perform a wide or full range of light work represents substantial work capability compatible with making a work adjustment to substantial numbers of unskilled jobs and, thus, generally provides sufficient occupational mobility even for severely impaired individuals who are not of advanced age and have sufficient educational competences for unskilled work.
(c) However, for individuals of advanced age who can no longer perform vocationally relevant past work and who have a history of unskilled work experience, or who have only skills that are not readily transferable to a significant range of semi-skilled or skilled work that is within the individual's functional capacity, or who have no work experience, the limitations in vocational adaptability represented by functional restriction to light work warrant a finding of disabled. Ordinarily, even a high school education or more which was completed in the remote past will have little positive impact on effecting a vocational adjustment unless relevant work experience reflects use of such education.
(d) A finding of disabled is warranted where the same factors in paragraph (c) of this section regarding education and previous work experience are present, but where age, though not advanced, is a factor which significantly limits vocational adaptability (i.e., closely approaching advanced age, 50-54) and an individual's vocational scope is further significantly limited by illiteracy.
(e) The presence of acquired skills that are readily transferable to a significant range of semi-skilled or skilled work within an individual's residual functional capacity would ordinarily warrant a finding of not disabled regardless of the adversity of age, or whether the individual's formal education is commensurate with his or her demonstrated skill level. The acquisition of work skills demonstrates the ability to perform work at the level of complexity demonstrated by the skill level attained regardless of the individual's formal educational attainments.
(f) For a finding of transferability of skills to light work for persons of advanced age who are closely approaching retirement age (age 60 or older), there must be very little, if any, vocational adjustment required in terms of tools, work processes, work settings, or the industry.
(g) While illiteracy may significantly limit an individual's vocational scope, the primary work functions in most unskilled occupations relate to working with things (rather than data or people). In these work functions, education has the least significance. Similarly, the lack of relevant work experience would have little significance since the bulk of unskilled jobs require no qualifying work experience. The capability for light work, which includes the ability to do sedentary work, represents the capability for substantial numbers of such jobs. This, in turn, represents substantial vocational scope for younger individuals (age 18-49), even if they are illiterate.
Table No. 2 - Residual Functional Capacity: Maximum Sustained Work Capability Limited to Light Work as a Result of Severe Medically Determinable Impairment(s)
| Rule | Age | Education | Previous work experience | Decision |
|---|---|---|---|---|
| 202.01 | Advanced age | Limited or less | Unskilled or none | Disabled. |
| 202.02 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 202.03 | ......do | ......do | Skilled or semiskilled - skills transferable 1 | Not disabled. |
| 202.04 | ......do | High school graduate or more - does not provide for direct entry into skilled work 2 | Unskilled or none | Disabled. |
| 202.05 | ......do | High school graduate or more - provides for direct entry into skilled work 2 | ......do | Not disabled. |
| 202.06 | ......do | High school graduate or more - does not provide for direct entry into skilled work 2 | Skilled or semiskilled - skills not transferable | Disabled. |
| 202.07 | ......do | ......do | Skilled or semiskilled - skills transferable 2 | Not disabled. |
| 202.08 | ......do | High school graduate or more - provides for direct entry into skilled work 2 | Skilled or semiskilled - skills not transferable | Do. |
| 202.09 | Closely approaching advanced age | Illiterate | Unskilled or none | Disabled. |
| 202.10 | ......do | Limited or Marginal, but not Illiterate | ......do | Not disabled. |
| 202.11 | ......do | Limited or less | Skilled or semiskilled - skills not transferable | Do. |
| 202.12 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 202.13 | ......do | High school graduate or more | Unskilled or none | Do. |
| 202.14 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 202.15 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 202.16 | Younger individual | Illiterate | Unskilled or none | Do. |
| 202.17 | ......do | Limited or Marginal, but not Illiterate | ......do | Do. |
| 202.18 | ......do | Limited or less | Skilled or semiskilled - skills not transferable | Do. |
| 202.19 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 202.20 | ......do | High school graduate or more | Unskilled or none | Do. |
| 202.21 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 202.22 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
1 See 202.00(f).
2 See 202.00(c).
203.00 Maximum sustained work capability limited to medium work as a result of severe medically determinable impairment(s). (a) The functional capacity to perform medium work includes the functional capacity to perform sedentary, light, and medium work. Approximately 2,500 separate sedentary, light, and medium occupations can be identified, each occupation representing numerous jobs in the national economy which do not require skills or previous experience and which can be performed after a short demonstration or within 30 days.
(b) The functional capacity to perform medium work represents such substantial work capability at even the unskilled level that a finding of disabled is ordinarily not warranted in cases where a severely impaired person retains the functional capacity to perform medium work. Even the adversity of advanced age (55 or over) and a work history of unskilled work may be offset by the substantial work capability represented by the functional capacity to perform medium work. However, we will find that a person who (1) has a marginal education, (2) has work experience of 35 years or more doing only arduous unskilled physical labor, (3) is not working, and (4) is no longer able to do this kind of work because of a severe impairment(s) is disabled, even though the person is able to do medium work. (See § 404.1562(a) in this subpart and § 416.962(a) in subpart I of part 416.)
(c) However, the absence of any relevant work experience becomes a more significant adversity for persons of advanced age (55 and over). Accordingly, this factor, in combination with a limited education or less, militates against making a vocational adjustment to even this substantial range of work and a finding of disabled is appropriate. Further, for persons closely approaching retirement age (60 or older) with a work history of unskilled work and with marginal education or less, a finding of disabled is appropriate.
Table No. 3 - Residual Functional Capacity: Maximum Sustained Work Capability Limited to Medium Work as a Result of Severe Medically Determinable Impairment(s)
| Rule | Age | Education | Previous work experience | Decision |
|---|---|---|---|---|
| 203.01 | Closely approaching retirement age | Marginal or Illiterate | Unskilled or none | Disabled. |
| 203.02 | ......do | Limited or less | None | Do. |
| 203.03 | ......do | Limited | Unskilled | Not disabled. |
| 203.04 | ......do | Limited or less | Skilled or semiskilled - skills not transferable | Do. |
| 203.05 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.06 | ......do | High school graduate or more | Unskilled or none | Do. |
| 203.07 | ......do | High school graduate or more - does not provide for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.08 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.09 | ......do | High school graduate or more - provides for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.10 | Advanced age | Limited or less | None | Disabled. |
| 203.11 | ......do | ......do | Unskilled | Not disabled. |
| 203.12 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 203.13 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.14 | ......do | High school graduate or more | Unskilled or none | Do. |
| 203.15 | ......do | High school graduate or more - does not provide for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.16 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.17 | ......do | High school graduate or more - provides for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.18 | Closely approaching advanced age | Limited or less | Unskilled or none | Do. |
| 203.19 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 203.20 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.21 | ......do | High school graduate or more | Unskilled or none | Do. |
| 203.22 | ......do | High school graduate or more - does not provide for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.23 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.24 | ......do | High school graduate or more - provides for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.25 | Younger individual | Limited or less | Unskilled or none | Do. |
| 203.26 | ......do | ......do | Skilled or semiskilled - skills not transferable | Do. |
| 203.27 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.28 | ......do | High school graduate or more | Unskilled or none | Do. |
| 203.29 | ......do | High school graduate or more - does not provide for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
| 203.30 | ......do | ......do | Skilled or semiskilled - skills transferable | Do. |
| 203.31 | ......do | High school graduate or more - provides for direct entry into skilled work | Skilled or semiskilled - skills not transferable | Do. |
204.00 Maximum sustained work capability limited to heavy work (or very heavy work) as a result of severe medically determinable impairment(s). The residual functional capacity to perform heavy work or very heavy work includes the functional capability for work at the lesser functional levels as well, and represents substantial work capability for jobs in the national economy at all skill and physical demand levels. Individuals who retain the functional capacity to perform heavy work (or very heavy work) ordinarily will not have a severe impairment or will be able to do their past work - either of which would have already provided a basis for a decision of “not disabled”. Environmental restrictions ordinarily would not significantly affect the range of work existing in the national economy for individuals with the physical capability for heavy work (or very heavy work). Thus an impairment which does not preclude heavy work (or very heavy work) would not ordinarily be the primary reason for unemployment, and generally is sufficient for a finding of not disabled, even though age, education, and skill level of prior work experience may be considered adverse.
[45 FR 55584, Aug. 20, 1980, as amended at 56 FR 57944, Nov. 14, 1991; 68 FR 51164, Aug. 26, 2003; 73 FR 64197, Oct. 29, 2008; 85 FR 10602, Feb. 25, 2020]