Title 21
SECTION 862.1385
862.1385 17-Hydroxycorticosteroids (17-ketogenic steroids) test system.
§ 862.1385 17-Hydroxycorticosteroids (17-ketogenic steroids) test system.(a) Identification. A 17-hydroxycorticosteroids (17-ketogenic steroids) test system is a device intended to measure corticosteroids that possess a dihydroxyacetone
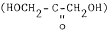 moiety on
the steroid nucleus in urine. Corticosteroids with this chemical
configuration include cortisol, cortisone 11-desoxycortisol,
desoxycorticosterone, and their tetrahydroderivatives. This group
of hormones is synthesized by the adrenal gland. Measurements of
17-hydroxycorticosteroids (17-ketogenic steroids) are used in the
diagnosis and treatment of various diseases of the adrenal or
pituitary glands and gonadal disorders.
moiety on
the steroid nucleus in urine. Corticosteroids with this chemical
configuration include cortisol, cortisone 11-desoxycortisol,
desoxycorticosterone, and their tetrahydroderivatives. This group
of hormones is synthesized by the adrenal gland. Measurements of
17-hydroxycorticosteroids (17-ketogenic steroids) are used in the
diagnosis and treatment of various diseases of the adrenal or
pituitary glands and gonadal disorders.
(b) Classification. Class I (general controls). The device is exempt from the premarket notification procedures in subpart E of part 807 of this chapter subject to § 862.9.
[52 FR 16122, May 1, 1987; 52 FR 29468, Aug. 7, 1987, as amended at 65 FR 2306, Jan. 14, 2000]